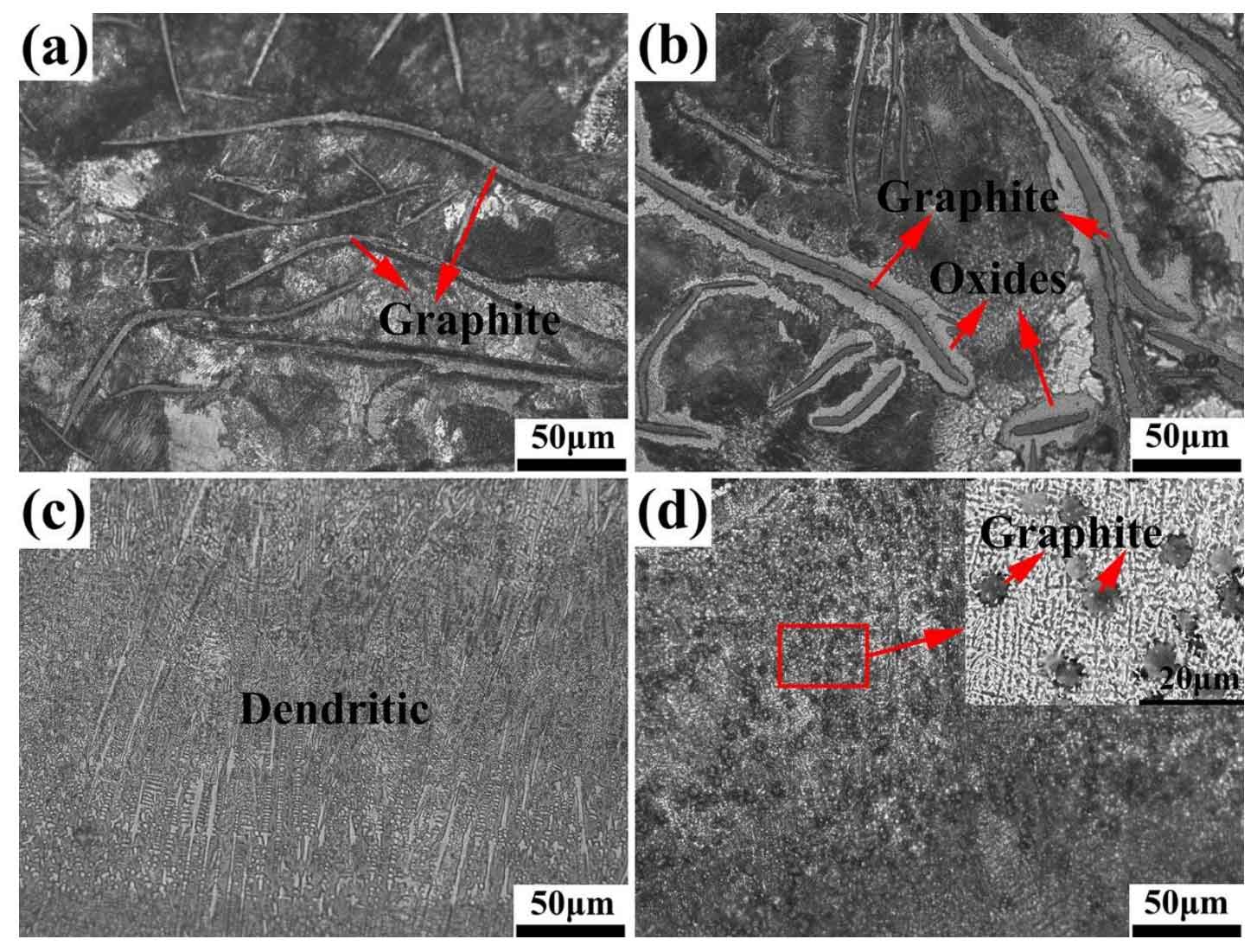
Figure 1 shows the microstructure changes of gray cast iron matrix and unit without thermal fatigue and 300 thermal cycles. In the process of thermal cycle, because most of the graphite and the matrix are in a non coherent state, and the physical properties such as thermal conductivity and thermal expansion coefficient are different, repeated heating and cooling make a gap between the graphite and the matrix. Due to the outward diffusion of graphite and the infiltration of oxygen into the matrix through the phase interface during the thermal cycle. Continuous thermal expansion and cold contraction expand the gap between the matrix and graphite, so that oxygen can enter the matrix along the graphite gap.
(b) matrix morphologyafter 300 thermal cycles;
(c) unit morphology of pre-thermal cycle;
(d) unit morphology after 300 thermal cycles
Therefore, oxygen reacts with iron around graphite to form oxide around graphite and form a loose structure around the outer layer of graphite. The matrix is mainly pearlite structure, which is a lamellar structure composed of ferrite and cementite (as shown in Fig. 1 (b)). Cementite belongs to metastable structure, which is easy to decompose in the process of thermal cycle, so that the pearlite in the matrix is gradually broken and the content is reduced. The unit body is fine dendrite structure before thermal fatigue of gray cast iron. According to XRD diffraction analysis, we know that there is a large amount of white mouth structure of cementite in it. In the process of thermal cycle, a large number of cementite in the matrix decomposes, and the precipitated carbon enriches to the surface layer to form a decarburization layer; On the other hand, it is enriched in the unit body to form spherical graphite (as shown in Fig. 1 (d)), while the dendrites in the unit body area are gradually broken.
In the process of thermal cycle, the hardness also changes due to the change of matrix structure. As shown in Figure 2 (a), the hardness change curve of the unit body on the worn surface of the sample is shown. With the increase of carbon content, the carbon dissolved in the unit body in the laser melting process is also higher and higher, which increases the content of carbon iron compound as hard phase in the unit body. Therefore, the average hardness of the unit body of non gray cast iron after thermal fatigue treatment increases from 192.7 hv0.5-260 hv0.5 of the matrix to 677 hv0.5-792.9 hv0.5 of the unit body. According to the analysis in the above paragraph, the carbon iron compound in the unit body is cementite. In the process of thermal cycle, cementite with metastable structure changes to ferrite and graphite with stable structure, which makes cementite decompose a lot; After 300 thermal cycles, the hardness decreases by about 150-200hv0.5. As shown in Fig. 2 (a), the change of unit hardness curve from untreated unit hardness curve after 150 thermal cycles to unit hardness curve after 300 thermal cycles is shown.
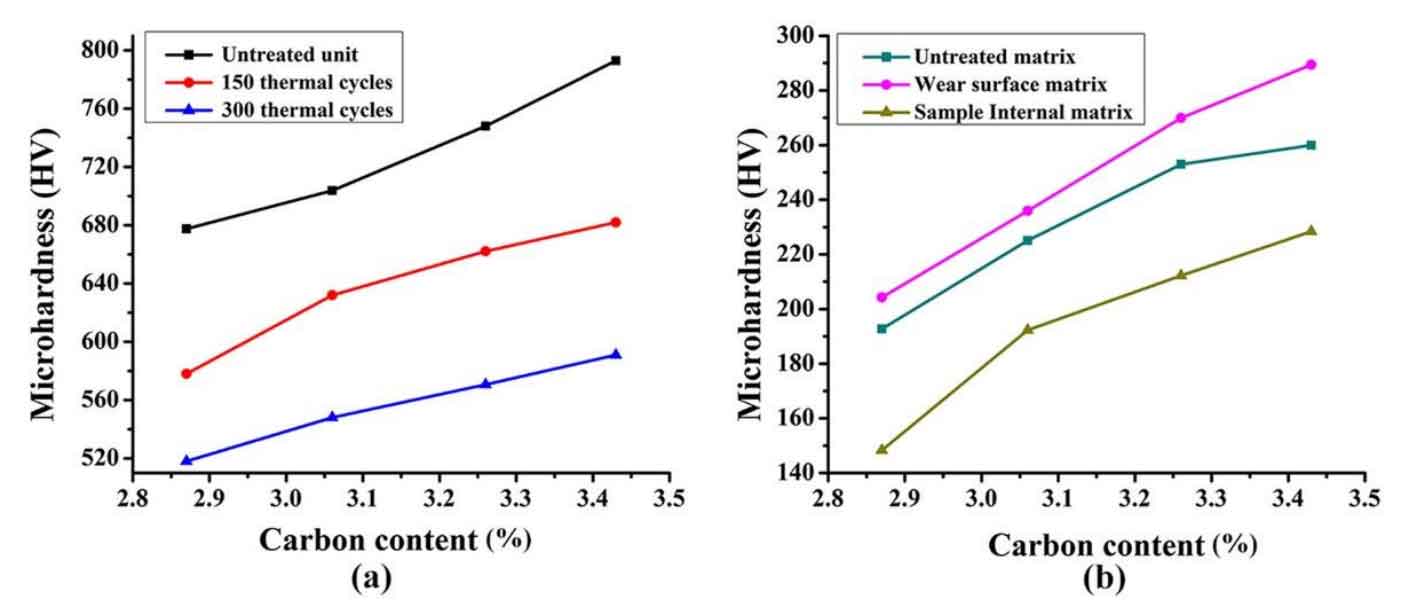
The change of matrix hardness is similar to that of unit body. For example, the matrix hardness of untreated samples increases gradually with the increase of carbon content. In the process of thermal fatigue of gray cast iron, pearlite in the matrix is gradually decomposed, some carbon is enriched and precipitated to the surface of the sample, and some exist in the matrix as free graphite. Therefore, the average hardness in the matrix after thermal fatigue of gray cast iron is lower than that before thermal fatigue of gray cast iron (as shown in Figure 2 (b)). However, the hardness of the surface matrix after wear is slightly different. On the one hand, the reason may be that the work hardening of the sample surface occurs during the friction process; On the other hand, it may be due to the rapid cooling rate during the thermal cycle, resulting in martensite. For these reasons, the surface hardness of the sample after thermal fatigue is slightly higher than that of the matrix before thermal fatigue.
