In my extensive experience in the foundry industry, I have dedicated much of my career to refining processes for producing high-quality shell castings. Shell castings, particularly for complex components like pump housings, valve bodies, and engine blocks, require meticulous attention to detail in every stage—from mold preparation and core making to melting, pouring, and post-casting treatments. The demand for shell castings with intricate internal channels, superior mechanical properties, and dimensional accuracy has driven innovations in materials and methods. Here, I will share insights from practical applications, focusing on copper-alloy and aluminum-alloy shell castings, as well as advancements in cleaning techniques like water explosion descaling for steel castings. Throughout this discussion, the term “shell castings” will be emphasized to highlight its centrality in modern manufacturing.
My journey with shell castings began with copper-based alloys, which are prized for their strength, corrosion resistance, and ease of casting. For instance, in producing large copper shell castings, such as those for marine components or industrial machinery, the melting process is critical. We often use a triple-crucible furnace setup to handle substantial volumes. The charge sequence typically involves cold loading copper, metallic manganese, and low-carbon steel scraps, followed by covering with charcoal. Once melted, the charcoal is skimmed off, and preheated zinc and tin are added, with aluminum introduced last. This method ensures uniform composition and minimizes oxidation. The entire melting cycle, from air-blowing to completion, takes approximately 4 to 6 hours, depending on the batch size. After melting, the molten copper is transferred to a preheated tapping ladle, slag is removed, and grass ash is added as a cover. Pouring occurs at around 1150–1200°C to fill the mold cavity completely. For shell castings with open-top designs, we place a red-hot furnace lid over the casting surface to promote slow cooling, reducing shrinkage defects.
| Material | Amount (kg) | Percentage (%) | Role in Shell Castings |
|---|---|---|---|
| Electrolytic Copper | 150–200 | 60–70 | Base metal providing conductivity and strength |
| Zinc Ingot | 50–80 | 20–30 | Enhances fluidity and corrosion resistance |
| Aluminum Ingot | 10–20 | 5–10 | Improves hardness and oxidation resistance |
| Tin | 5–10 | 2–5 | Increases wear resistance and castability |
| Metallic Manganese | 3–7 | 1–3 | Strengthens alloy and refines grain structure |
| Low-Carbon Steel Scrap | 20–30 | 8–12 | Adds toughness and reduces cost |
The chemical analysis of the final shell castings often shows slight deviations, such as higher manganese and lower iron content than specified, but this does not compromise performance. Tensile test samples cut from the runners exhibit impressive as-cast mechanical properties: tensile strength (σ_b) of 450–550 MPa, yield strength (σ_s) of 250–300 MPa, and elongation (δ) of 15–25%. These values underscore the reliability of this melting approach for shell castings. The strength and ductility can be modeled using empirical formulas. For example, the ultimate tensile strength for copper-alloy shell castings can be estimated as:
$$ \sigma_b = C_0 + k_1 \cdot \text{Cu\%} + k_2 \cdot \text{Zn\%} + k_3 \cdot \text{Al\%} $$
where \( C_0 \) is a base constant, and \( k_1, k_2, k_3 \) are coefficients derived from regression analysis. Similarly, the melting time \( t_m \) for shell castings in a crucible furnace relates to the charge weight \( W \) and furnace power \( P \):
$$ t_m = \frac{W \cdot C_p \cdot \Delta T}{\eta \cdot P} $$
Here, \( C_p \) is the specific heat capacity, \( \Delta T \) is the temperature rise, and \( \eta \) is the thermal efficiency. These equations help optimize processes for shell castings, ensuring consistent quality.
However, one drawback of this method for shell castings is the relatively low yield rate and excessive machining allowances. We are exploring sequential solidification techniques to minimize upper-surface machining, saving material and labor. This aligns with the broader goal of enhancing efficiency in shell castings production.
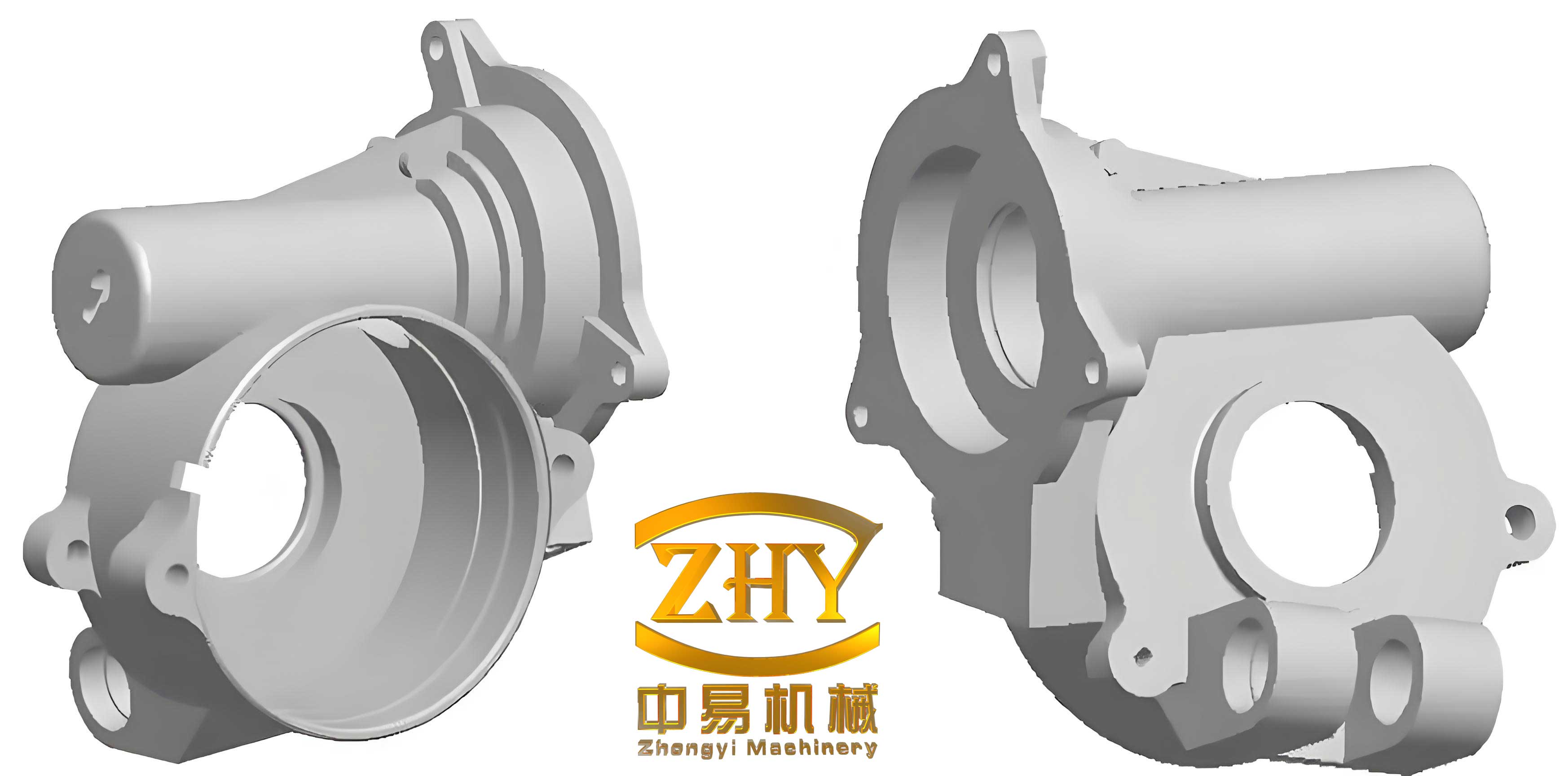
Transitioning to aluminum-alloy shell castings, these are increasingly used for components requiring lightweight yet robust structures with complex internal passages. In my work, I have focused on casting oil passages directly into aluminum housings for valves and pumps, eliminating the need for secondary machining. For shell castings with fine channels as small as 2–3 mm in diameter, core-making is paramount. We use a variety of materials, including sand cores, copper tubes, and steel wires, tailored to the geometry. A common mix for sand cores in aluminum shell castings involves silica sand (AFS grain fineness 70–100), bonded with a blend of molasses, tung oil, and water. The core sand mixture is prepared by first dissolving molasses in water, then adding silica sand in a mixer, followed by tung oil, and mixing for 8–10 minutes. The resulting properties are crucial for shell castings: wet compressive strength of 0.4–0.6 kg/cm² and permeability over 100.
| Ingredient | Proportion (by weight) | Function in Shell Castings |
|---|---|---|
| Silica Sand (70–100 AFS) | 100 parts | Refractory base for core stability |
| Molasses | 3–5 parts | Binder providing green strength |
| Tung Oil | 1–2 parts | Enhances dry strength and collapsibility |
| Water | 4–6 parts | Adjusts viscosity and workability |
Baking temperatures for these cores in shell castings depend on size and complexity. For a typical aluminum housing core, we use a stepped profile: ramp up to 180°C over 2 hours, hold for 1–2 hours, then cool slowly. This prevents cracking and ensures adequate strength. To create rectangular passages of 3 mm × 5 mm cross-section, we embed 3 mm diameter copper tubes that serve dual roles as cores and vents. These tubes are coated with a molybdenum disulfide-alcohol paste to ease removal after casting. For even finer 2 mm diameter holes, we employ bright steel wires bent to shape, coated similarly, and pulled out post-casting. In cases where cores are inaccessible, such as internal labyrinths, we use copper strips dissolved later with nitric acid, a technique that preserves the integrity of shell castings.
Core assembly for multi-passage shell castings requires precision. We avoid excessive joints to reduce errors, but for intersecting oil ways, bonding is necessary. A typical adhesive consists of waste sulfite liquor, clay, and water. For non-machined seams, a paste of graphite powder, clay, and salt is applied. Each joint is dried under infrared lamps to prevent displacement. The entire core assembly is then baked according to the profile mentioned. Quality checks for these shell castings include visual inspection and X-ray examination to rule out shrinkage, porosity, or misruns. Pressure testing at 50–60 kg/cm² confirms leak-tight performance, demonstrating the viability of cast-in passages for shell castings.
The advantages of this approach for shell castings are manifold: reduced part weight, simplified machining, fewer tooling requirements, and shorter lead times. Compared to traditional methods, it allows for more compact and efficient designs. In my practice, I have seen shell castings with such integrated channels outperform machined counterparts in durability and cost-effectiveness.
Another critical aspect of shell castings production is post-casting cleaning, especially for steel components. Water explosion descaling (water爆清砂) is a technique I have adapted for various alloys, including carbon and manganese steels. By immersing hot castings in water, thermal shock removes sand and scale rapidly. The key is timing the shakeout to avoid cracking. Based on my trials, I have developed a reference chart for open-time after pouring for shell castings using sodium silicate-bonded sand. This chart correlates casting weight, section thickness, and steel grade to recommended shakeout times. For dry clay-sand molds, times should be extended by 20–30%. Susceptible shell castings, like those with uneven sections, require longer intervals.
| Casting Weight (kg) | Wall Thickness (mm) | Carbon Steel (hours) | Manganese Steel (hours) | Alloy Steel (hours) |
|---|---|---|---|---|
| 10–50 | 10–20 | 1–2 | 2–3 | 2–4 |
| 50–200 | 20–40 | 2–4 | 3–5 | 4–6 |
| 200–500 | 40–60 | 4–6 | 5–7 | 6–8 |
| 500–1000 | 60–100 | 6–10 | 8–12 | 10–14 |
The relationship between shakeout time \( t_s \) and casting parameters for shell castings can be approximated by:
$$ t_s = A \cdot W^{0.5} + B \cdot T^{1.2} $$
where \( W \) is weight in kg, \( T \) is thickness in mm, and \( A, B \) are material-specific constants (e.g., for carbon steel shell castings, \( A \approx 0.1 \), \( B \approx 0.05 \)). Low-temperature water explosion, at around 300–400°C, has proven effective for shell castings, minimizing residual stresses while ensuring thorough cleaning. This method has improved labor conditions and efficiency in my foundry, much like the triple-crucible setup for copper shell castings.
Beyond these specific processes, I have explored broader innovations in shell castings. For instance, simulation software helps predict solidification patterns and defect formation in shell castings. Using finite element analysis, we can model temperature gradients and optimize gating systems. The governing heat transfer equation during solidification of shell castings is:
$$ \rho C_p \frac{\partial T}{\partial t} = \nabla \cdot (k \nabla T) + L \frac{\partial f_s}{\partial t} $$
Here, \( \rho \) is density, \( k \) is thermal conductivity, \( L \) is latent heat, and \( f_s \) is solid fraction. Such tools enable us to design shell castings with improved yield and reduced scrap.
Material science also plays a role. For high-performance shell castings, we experiment with novel alloys, such as aluminum-silicon-magnesium systems for automotive parts or copper-nickel-tin for marine applications. The hardness \( H \) of aluminum shell castings can be related to silicon content \( \text{Si\%} \) and aging time \( t_a \):
$$ H = H_0 + \alpha \cdot \text{Si\%} – \beta \cdot \ln(t_a + 1) $$
where \( H_0, \alpha, \beta \) are empirical coefficients. These relationships guide alloy selection for specific shell castings requirements.
In terms of sustainability, shell castings offer opportunities for recycling. We often revert scrap shell castings into the melt, adjusting charges to maintain chemistry. The recycling rate \( R \) for aluminum shell castings can be expressed as:
$$ R = \frac{M_{\text{recycled}}}{M_{\text{total}}} \times 100\% $$
With proper control, we achieve rates over 80%, reducing environmental impact. This circular approach is integral to modern shell castings production.
Looking ahead, challenges remain for shell castings, such as achieving thinner walls or higher precision. Additive manufacturing of cores and molds is an emerging trend. By 3D-printing sand cores, we can create previously impossible geometries for shell castings, such as undercuts or multi-branch channels. This aligns with the industry’s push toward digital foundries. In my projects, I have prototyped such cores for aerospace shell castings, cutting lead times by half while enhancing complexity.
Training and skill development are also vital. I mentor apprentices on the nuances of shell castings, emphasizing hands-on practice with melting, core-making, and quality control. The human element ensures that technological advances are applied effectively. For example, understanding the “feel” of sand consistency or the visual cues of molten metal temperature is irreplaceable in producing reliable shell castings.
In conclusion, my journey with shell castings has been one of continuous learning and innovation. From copper-alloy melting techniques to aluminum oil-passage casting and steel descaling methods, each step contributes to better, more efficient components. Shell castings, with their versatility and performance, remain at the forefront of casting technology. By integrating empirical data, mathematical models, and practical wisdom, we can overcome limitations and unlock new potentials. I am excited to see how shell castings will evolve with materials science and digital tools, driving progress across industries from automotive to energy. As I reflect, the key takeaway is that shell castings are not just products but enablers of engineering excellence, and their continued refinement will shape the future of manufacturing.
To encapsulate, the tables and formulas provided here summarize critical aspects of shell castings production. They serve as a reference for practitioners aiming to optimize their processes. Whether dealing with copper, aluminum, or steel shell castings, the principles of careful material selection, controlled processing, and thorough validation hold true. I encourage fellow engineers to experiment and share findings, fostering a collaborative community around shell castings. Together, we can push the boundaries of what is possible, ensuring that shell castings meet the ever-growing demands of modern technology.
