There may be two reasons for this phenomenon: holding time of molten iron and water content of molding sand.
1. Holding time of molten iron
When duplex smelting is adopted in general factories, for the sake of cost saving, for example, when the production process is not stopped for a long time, the molten iron in the holding electric furnace will be kept warm for a period of time, sometimes up to about 10 h. because the molten iron will change under the action of induction current stirring in the electric furnace for a long time, such as fine crystalline graphite and foreign crystalline core will gradually dissolve in the molten iron, The graphitized core is greatly reduced. This change of molten iron will lead to large undercooling, and the conventional inoculation treatment can not obtain the required microstructure; At the same time, it is also found that even if the chemical composition fully meets the requirements, the internal quality of the cast blank is still unqualified, and a large amount of ferrite and undercooled graphite will be produced. This should be the reason why there is a ferrite layer of about 1 ~ 2 mm on the overall surface of the gray cast iron cylinder head, that is, the long holding time of molten iron may reduce the hardness of the overall surface and even the core of the cylinder head.
2. Effect of molding sand water content on surface hardness
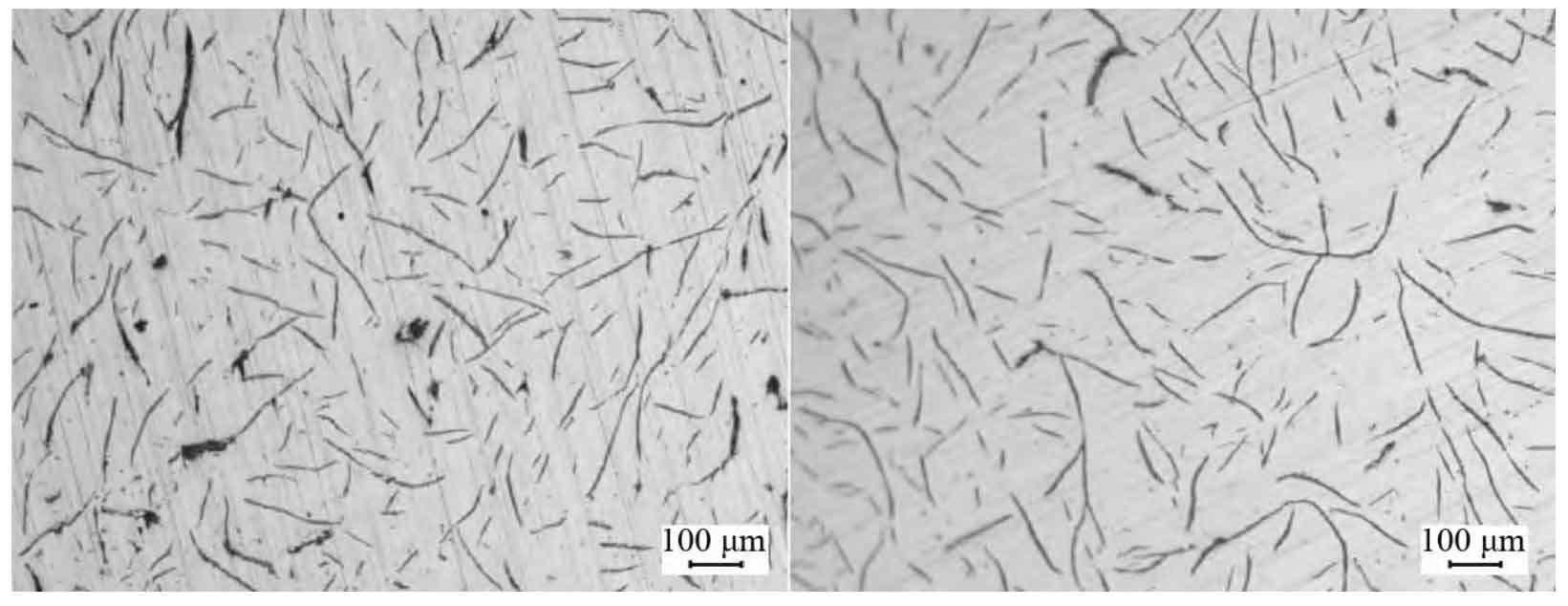
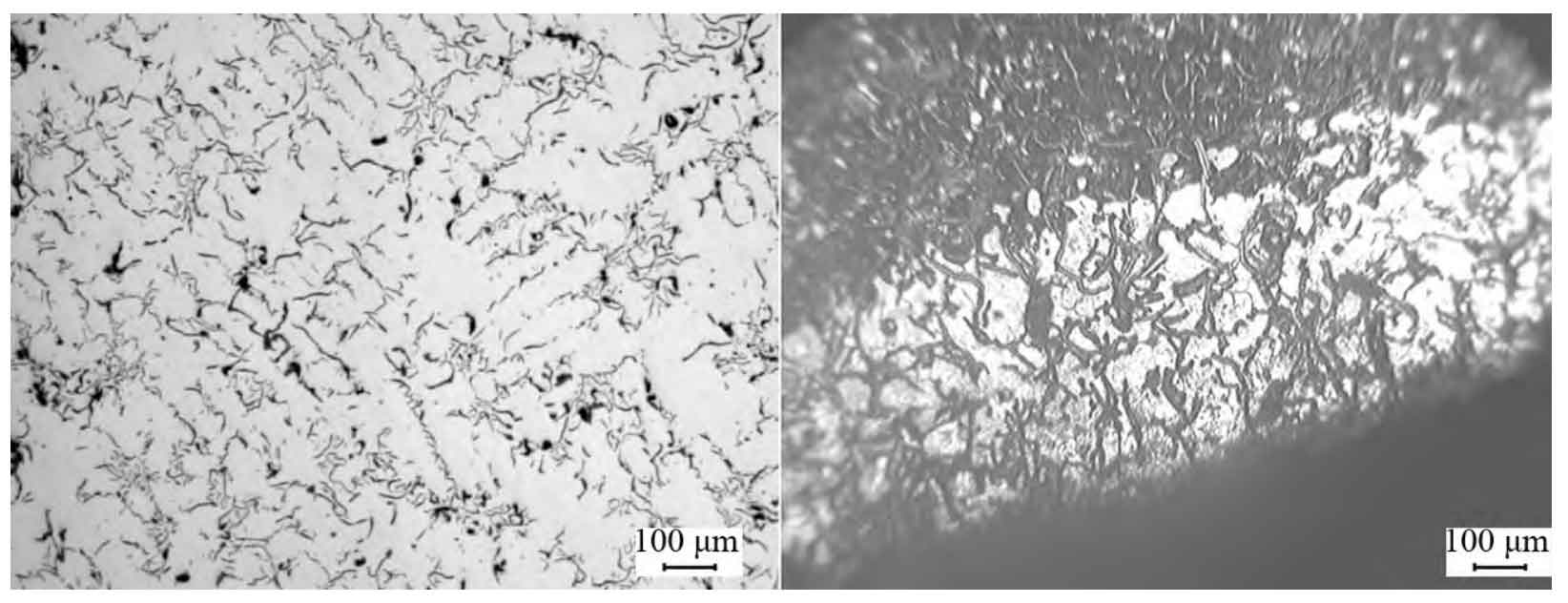
The moisture content of molding sand is controlled according to two levels: the low level is 3.0% ~ 3.4%, and the high level is 3.6% ~ 4.0%. The test results of the products produced by the two test schemes are shown in the table. The average hardness of the products in scheme 1 is HB 213. The surface graphite morphology and matrix structure are shown in Fig. 1a and Fig. 1b, both of which are type a graphite and pearlite with a content of 95%. The average hardness of the product in scheme 2 is HB 189, and there is an obvious ferrite layer on the surface metallographic structure. The thickness of the ferrite layer is about 1 ~ 2 mm, as shown in Fig. 2B. At the same time, it is also found that there is a large amount of e-type supercooled graphite in the ferrite layer area, as shown in Fig. 2A. After polishing the product surface of scheme 2, confirm the metallography again. Like scheme 1, the content of type a graphite and pearlite is 95%, indicating that the reason affecting the low hardness of scheme 1 should be the surface ferrite layer.
| Programme | Average surface hardness HB | Water content control level of molding sand | Tensile strength / MPa |
| 1 | 213 | low-level | 264 |
| 2 | 189 | high-level | 267 |
The metallographic sample in scheme 2 is the main bolt hole, which is the surface formed by the mold. Then, it is found that there is no obvious ferrite layer on the bottom surface of the combustion chamber of this part, which is the surface formed by the sand core. The ferrite layer is easy to appear in the gray cast iron cylinder head formed by molding sand, which may be the main reason why there is an obvious ferrite layer at the main bolt hole of the same gray cast iron cylinder head, but there is no ferrite layer on the bottom of the combustion chamber. Because the ferrite layer only appears on the body surface in contact with the molding sand, the influence of the molding sand on the gray cast iron cylinder head is mainly considered in the analysis.
Firstly, the green molding sand is mixed with a certain proportion of pulverized coal, so that the carbon element close to the surface of the molding sand is extremely rich. At the same time, the silicon element increases the carbon activity and force. As a graphitization element, the silicon can also reach more than 1.9% in the gray cast iron cylinder head. After the molten iron is poured, the reaction of the molten iron layer close to the sand core in the mold cavity is strengthened, the decarburization is significant, and the formation of ferrite can be significantly promoted. Silicon is an element with strong segregation tendency. Because the early solidification layer of gray cast iron cylinder head is rich in silicon, which reduces the solubility of carbon in molten iron, eutectoid transformation occurs in the cooling process of molten iron, austenite precipitates supersaturated carbon on nearby graphite, and the precipitation of graphite phase creates conditions for the nucleation and growth of ferrite, so as to make the matrix ferrite. Secondly, the high water content of molding sand leads to the change of local cooling environment. If the water content near the molding sand is high, it will increase the undercooling of molten iron on the mold surface. Type D and E are formed when the graphite nucleation conditions are poor and the cooling rate is high. The amount, shape and location of ferrite will be affected by austenite dendrite, so it is easy to understand that ferrite is associated with E-type graphite. The water content of molding sand is the reason that affects the ferrite layer on the contact surface of molding sand.