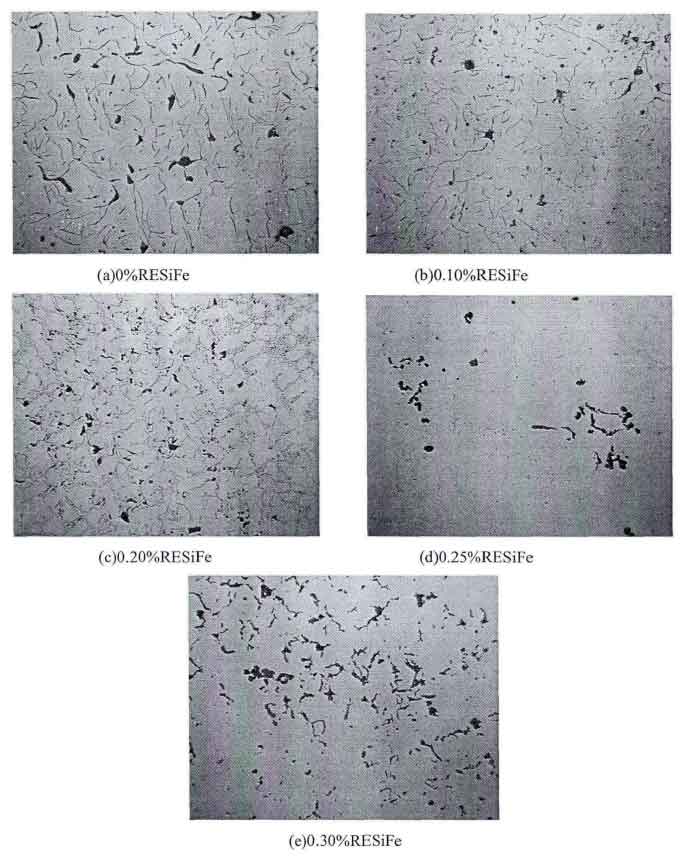
Comparing the graphite morphology of low carbon equivalent gray cast iron treated with different rare earth addition, it can be found (as shown in the figure)
- Before modification, the graphite morphology of low carbon equivalent gray cast iron is mainly E-type undercooled graphite, including a small amount of coarse A-type graphite, and the graphite sheet is long.
- After adding 01% rare earth alloy to gray cast iron, the graphite morphology is mostly flake A-type graphite, and a small amount of D-type and E-type supercooled graphite appears. The graphite flakes are fine and long, the number of graphite increases, and the distribution begins to tend to be uniform.
- When the addition of rare earth alloy in gray cast iron increases to 0.20%, the graphite morphology changes to dendritic point D-type graphite and E-type graphite. Undercooled graphite has accounted for the vast majority, and the number of graphite is still large.
- When the addition amount of rare earth alloy in gray cast iron is 0.25%, the graphite gathers, and the graphite form becomes flocculent. The graphite is thick and short, and the distribution is very uneven. The graphite is accumulated in some areas, so the number of graphite in the figure is small.
- Continue to increase the amount of rare earth alloy in gray cast iron to 0.3%, the graphite form in gray cast iron changes to worm like plus spherical graphite, in which the spherical graphite increases greatly and occupies most of the area, and the graphite distribution is relatively uniform.
It can be seen from the changes of graphite morphology and quantity that rare earth can promote the graphitization and double inoculation of low-carbon equivalent gray cast iron. When the addition amount of rare earth is 0.1%, the graphite morphology is optimized, the number of graphite increases and the graphite is refined. At this time, rare earth plays an obvious role in promoting. Rare earth also reacts with oxygen and sulfur in molten iron to form rare earth oxides and rare earth sulfides with high solution point, These high melting point compounds exist as the core of graphite nucleation, which promotes the graphitization of low carbon equivalent gray cast iron and increases the number of graphite; When the content of rare earth reaches 0.2%, the undercooling tendency of rare earth increases and undercooled graphite begins to appear in a large area. At this time, rare earth plays the opposite role, and the number of graphite decreases instead of increasing; When the addition of rare earth reaches 0.25%, graphite begins to creep and spheroidize, indicating that rare earth is also a good vermicular and spheroidizing agent.