The two kinds of MNS inclusion defects observed are formed on the surface of oxidized inclusions. According to the literature reports, it can be inferred that these two kinds of MNS inclusion defects belong to class I sulfide, which are often produced in liquid steel with low degree of deoxidation. They are generally spherical composite oxygen sulfide and disorderly distributed in steel. However, the specific mechanism of precipitation and growth of such MNS inclusion defects on the surface of oxidized inclusions is still controversial. Wakoh et al. Proposed a mechanism of heterogeneous nucleation and precipitation of sulfide inclusion defects on the surface of mno-sio2 oxide, while Oikawa et al. Proposed a mechanism of heterogeneous nucleation and precipitation of sulfide inclusion defects on the surface of TiO2 for steel castings deoxidized with Ti. According to the explanation put forward by wakoh et al., a certain amount of Mn and S elements will be dissolved in mno-sio2 oxide.
During the cooling and solidification of liquid steel, the solubility of Mn and S elements in mno-sio2 oxide will gradually decrease, resulting in the precipitation of two elements in mno-sio2 oxide in the form of MNS compound, forming MNS inclusions attached to the surface of mno-sio2 oxide. The observation results show that the size of oxides in MNS inclusion defects is very small, so the number of MNS inclusions that can be precipitated is very limited, so this explanation can not explain the reason for the high sulfide content in MNS inclusion defects.
Based on the research results of steel castings deoxidized with Ti, Oikawa et al proposed that the precipitation of MNS inclusion defects on the oxide surface is completed by two monoeutectic reactions. The reaction formulas of the two reactions are: L1 → Fe (s) + L3 and L1 → Fe (s) + L2 + L3, where L1, L2 and L3 represent liquid Fe, liquid MNS and liquid (Ti, Mn) O respectively. In the process of reaction, liquid (Ti, Mn) O precipitates at the liquid-solid interface of steel castings through monoeutectic reaction. Then, liquid MNS will precipitate in liquid steel with liquid (Ti, Mn) o as the nucleation substrate, and gradually coat the liquid (Ti, Mn) O in the heart to form MNS inclusions with core-shell structure. In the interpretation of Oikawa et al., MNS inclusions are precipitated from liquid steel, and oxides are only used as the nucleation substrate of MNS precipitation, which can explain the high content of sulfide in MNS inclusion defects. However, the inclusion defect of this explanation is that there is no direct evidence that liquid (Ti, Mn) O can be used as the heterogeneous nucleation substrate of liquid MNS.
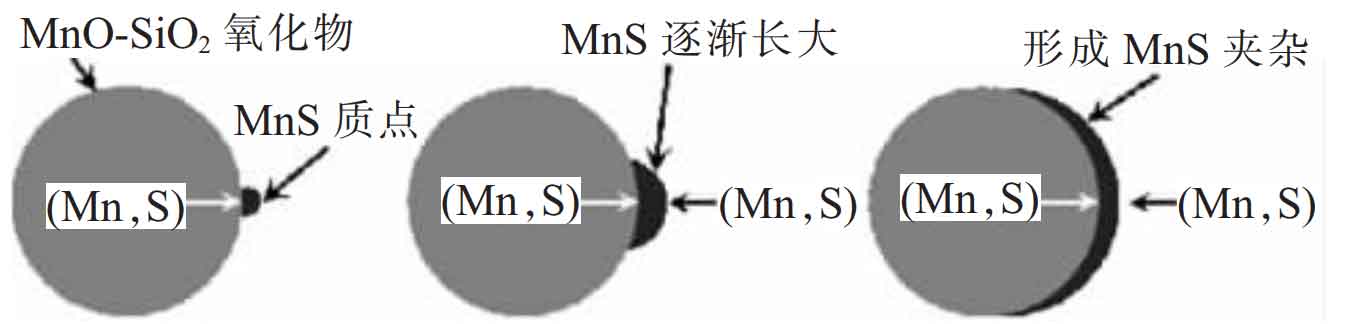
In order to more reasonably explain the nucleation and growth process of MNS inclusions on the oxide surface, Ma et al. Combined the explanation of wakoh et al. With the explanation of Oikawa et al., proposed the formation mechanism of class I MNS inclusions as shown in the figure. As shown in the figure, the formation of class I MNS inclusions in steel castings begins with the precipitation of MNs in the oxide, and the MNS precipitated in the oxide becomes the crystal nucleus for the continuous growth of MNS inclusions, while the Mn and S elements required for the continuous growth of MNS inclusions come from the Mn and s elements dissolved in the liquid steel. Therefore, the oxide itself is not a heterogeneous nucleation point precipitated by MNS, and the MNS nucleus precipitated on the oxide surface is the substrate for the nucleation and growth of MNS inclusions.