Abstract: This study investigates the effect of the addition of multi-scale ceramic particles (MSCP) on the wet corrosion resistance of QT400-18L nodular cast iron castings. By observing and testing the surface morphology, corrosion products, and corrosion rate of the samples under different humidities, it was found that MSCP can effectively improve the atmospheric corrosion resistance of nodular cast iron. This paper provides new insights into improving the corrosion resistance of nodular cast iron components used in coastal environments.
Keywords: QT400-18L; Multi-scale ceramic particles; Humidity; Corrosive properties; Nodular cast iron
1. Introduction
Wind turbines, especially those installed in coastal areas, are subjected to severe corrosion from seawater, tides, salt spray, and other natural environments. Key components such as wind turbine hubs, axles, and planetary supports are often produced using nodular cast iron QT400-18L due to its excellent mechanical properties. However, ensuring these components remain operational without maintenance or replacement for approximately 20 years poses significant challenges, particularly in terms of corrosion resistance.
This study focuses on enhancing the corrosion resistance of QT400-18L nodular cast iron by adding multi-scale ceramic particles (MSCP). The influence of MSCP on the corrosion behavior of the castings under varying humidity conditions was investigated, aiming to provide new insights for improving the corrosion resistance of nodular cast iron components used in coastal environments.
2. Materials and Methods
2.1 Material Composition
The chemical composition of QT400-18L nodular cast iron is as follows: w(C): 3.56%~3.68%, w(Si): 2.19%~2.40%, w(Mn): 0.148%~0.150%, w(P): 0.030%~0.032%, w(S): 0.010%~0.012%, w(Mg): 0.029%~0.062%, w(Re): 0.032%~0.047%, and the remainder is Fe.
2.2 MSCP Addition
The MSCP used in this study were activated multi-scale composite SiC particles added in the range of 0.05% to 0.15% (by mass). The particle size and surface area were characterized using a Mastersizer 2000 laser particle size analyzer.
2.3 Sample Preparation and Testing
Samples were taken from the center of poured test blocks and processed into disks with a diameter of 25 mm and a thickness of 3 mm. A full immersion test method was employed to measure the corrosion rate by determining the change in sample weight before and after immersion. The corrosion rate (V) was calculated using the formula:
V=AtM2−M1
where V is the corrosion rate in g/(m²·h), M1 and M2 are the masses of the sample before and after corrosion in g, A is the sample surface area in m², and t is the test duration in hours.
2.4 Microstructural and Corrosion Analysis
The surface morphology, corrosion product layer thickness, and composition of the corrosion products were observed using a Zeiss SUPRA 55 field emission scanning electron microscope (SEM) and energy-dispersive spectroscopy (EDS). X-ray diffraction (XRD) analysis was performed using an Empyrean X-ray diffractometer to determine the composition of the corrosion products. Image analysis software was utilized to analyze and calculate the graphite nodularity grade, graphite size, and matrix phase content according to GB/T 9441-2021.
3. Experimental Results and Analysis
3.1 Effect of MSCP on Graphite Morphology
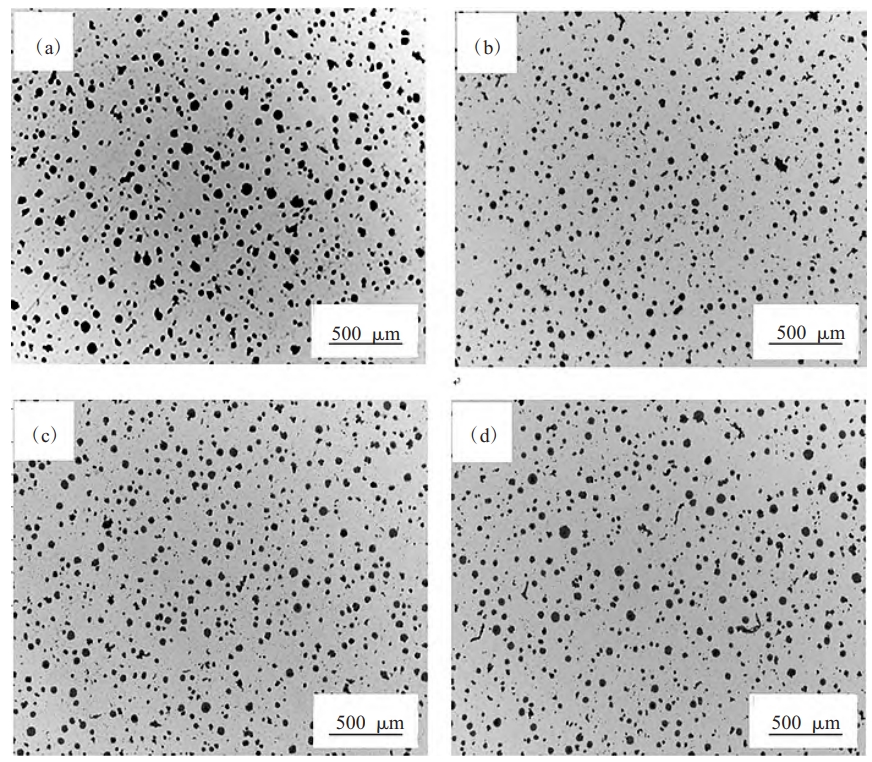
Table 2 illustrate the influence of MSCP addition on the graphite morphology of QT400-18L nodular cast iron. The results show that the graphite spheres in the samples without MSCP are uneven in size, with poor roundness and a small amount of vermicular graphite. In contrast, the addition of MSCP results in smaller, more rounded graphite spheres with improved nodularity and nodularization rates. The highest nodularity grade and best nodularization effect were observed in samples with an MSCP addition of 0.10%.
Table 1. MSCP Addition and Sample Preparation Details
| Sample No. | Humidity (%) | MSCP Addition (%) |
|---|---|---|
| 1-1# | 60 | 0.05 |
| 2-1# | 60 | 0.10 |
| 2-2# | 80 | 0.10 |
| … | … | … |
Table 2. Graphite Morphology after MSCP Addition
| MSCP Addition (%) | Nodularity Grade | Nodularization Rate | Graphite Size |
|---|---|---|---|
| 0 | 4 | 79% | 6 |
| 0.05 | 3 | 82% | 7 |
| 0.10 | 2 | 91% | 7 |
| 0.15 | – | 86% | – |
3.2 Effect of MSCP on Matrix Composition
The matrix composition of QT400-18L nodular cast iron with added MSCP. The matrix primarily consists of ferrite with a small amount of pearlite. Image analysis software revealed that the ferrite content in samples without MSCP was 79.16%, while samples with MSCP additions of 0.05%, 0.10%, and 0.15% had ferrite contents of 86.42%, 85.84%, and 86.95%, respectively. These results indicate that MSCP increases the ferrite content in the nodular cast iron.
3.3 Corrosion Rate Variation
The corrosion rate curves of QT400-18L nodular cast iron with and without MSCP under varying humidity conditions. The corrosion rate increases with humidity. Below 80% humidity, the corrosion rate increase is similar for both samples with and without MSCP. Above 80% humidity, the corrosion rate increases more rapidly, but the increase is less significant in samples with MSCP. At 90% and 98% humidity, the corrosion rates of samples with MSCP are lower than those without MSCP, with the lowest corrosion rate observed in samples with an MSCP addition of 0.15%.
3.4 Corrosion Product Analysis
The corrosion morphologies of samples after 168 hours at 80% humidity Samples without MSCP and with an MSCP addition of 0.05% exhibited the most severe corrosion, while those with additions of 0.10% and 0.15% showed less corrosion. The corrosion products are mainly iron oxides (FeO, Fe2O3, and Fe3O4), as confirmed by EDS and XRD analyses. The corrosion products show a characteristic “double ring” pattern, which is attributed to the formation of micro-droplets on the sample surface.
4. Discussion
4.1 Mechanism of Corrosion Product Formation
The formation of the “double ring” pattern of corrosion products observed in the study can be attributed to the micro-droplet phenomenon that occurs on the surface of the QT400-18L ductile iron castings. Under certain humidity conditions, atmospheric moisture preferentially adsorbs and accumulates on defective areas of the metal surface, where the activation energy is higher, facilitating the formation of small droplets. These primary droplets contain high concentrations of solutes that diffuse outwardly, resulting in the formation of micro-droplets with diameters ranging from 1 to 10 micrometers. These micro-droplets continue to spread and alter the local chemical environment of the metal material, thereby accelerating the atmospheric corrosion process.
The micro-droplets cover most of the ferrite-rich areas on the surface of the ductile iron castings. The ferrite has a lower electrode potential and acts as the anode in a galvanic cell, undergoing corrosion to form severe corrosion pits. The edges of these micro-droplets become cathodic regions, where the electrochemical corrosion process continues. As the corrosion progresses, the area between the two layers of rings becomes more activated, making it easier to adsorb water molecules from the atmosphere and transform into larger droplets, ultimately leading to the formation of more corrosion products in these regions. This explains the “double ring” feature observed in the corrosion products, with an inner and outer ring of corrosion products surrounding the graphite particles.
4.2 Corrosion Resistance Mechanism of Multi-Scale Ceramic Particles (MSCP)
The addition of multi-scale ceramic particles (MSCP) to QT400-18L ductile iron castings significantly improves their corrosion resistance, particularly in high-humidity environments. The corrosion resistance mechanism of MSCP can be attributed to several factors:
- Refinement and Modification of Graphite Structure: The addition of MSCP refines and modifies the graphite structure in the ductile iron, resulting in smaller, more spherical graphite particles with improved nodularity. This refinement and modification reduce the irregularity at the interface between graphite and the matrix, thereby lowering the energy available for corrosion development and diffusion. Consequently, the corrosion rate decreases.
- Increase in Ferrite Content: MSCP also increases the amount of ferrite in the microstructure of the ductile iron, which has a lower electrode potential compared to other phases. By increasing the ferrite content, the potential difference between the graphite (cathode) and the matrix (anode) is reduced, weakening the galvanic cell reaction and improving corrosion resistance.
- Formation of Protective Oxide Films: The activated MSCP contain metal elements that are prone to passivation, capable of forming strong and dense oxide films on the grain surfaces. These oxide films act as barriers to corrosion, further enhancing the corrosion resistance of the ductile iron castings.
- Modification of Microstructure: The uniform distribution of MSCP in the iron melt provides energy fluctuations and reduces the nucleation undercooling, resulting in a refined microstructure. This refined and dense microstructure hinders the corrosion of the metal matrix.
In conclusion, the addition of multi-scale ceramic particles effectively improves the corrosion resistance of QT400-18L ductile iron castings by refining and modifying the graphite structure, increasing the ferrite content, forming protective oxide films, and modifying the microstructure. These mechanisms collectively contribute to the enhanced corrosion performance of the castings, particularly in high-humidity environments such as coastal areas.
