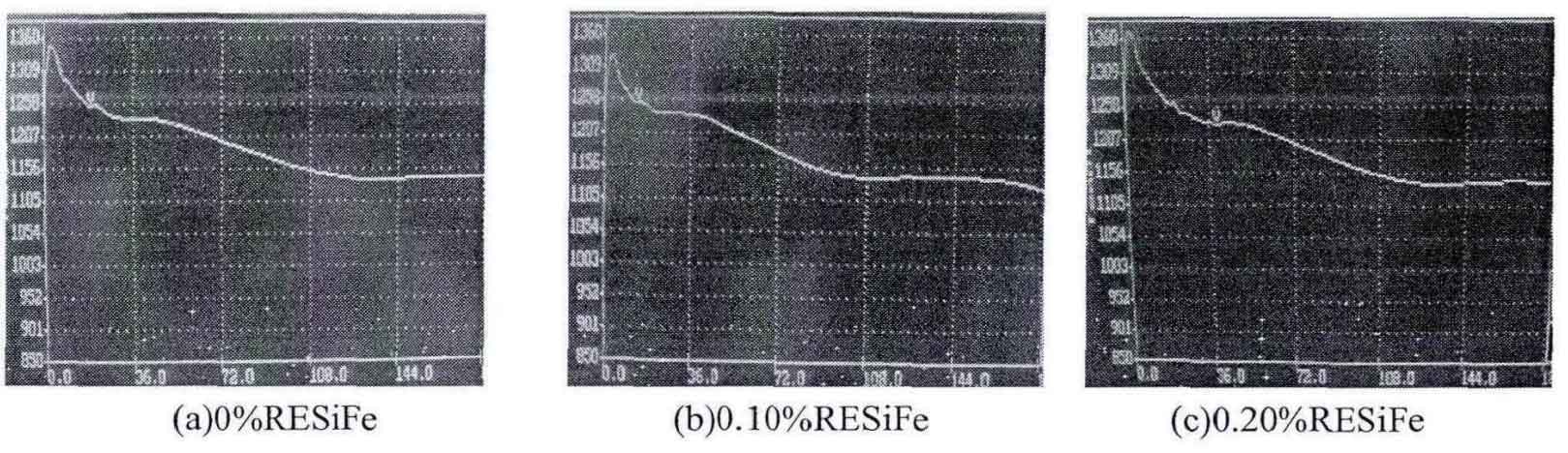
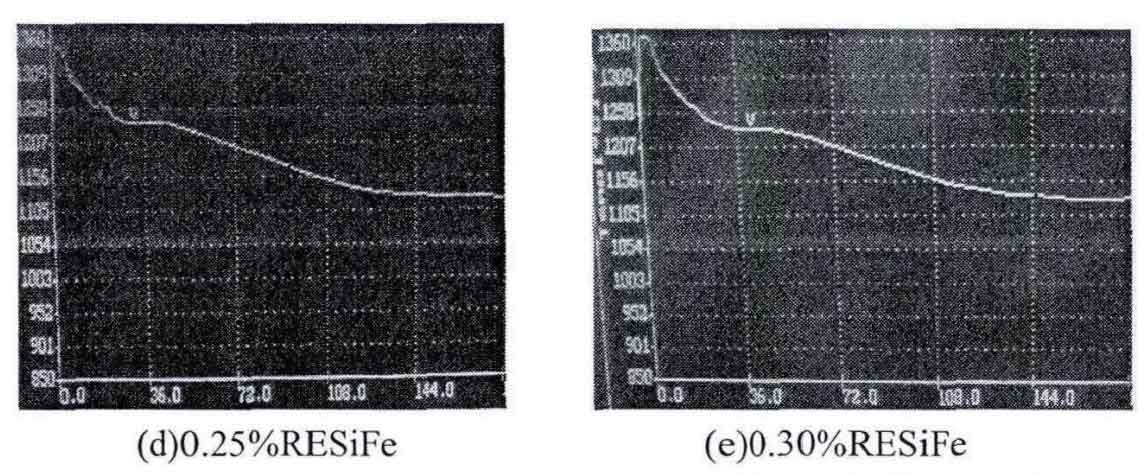
After the molten iron is poured into the crucible, the temperature of gray cast iron has a stable process, that is, a long eutectic platform appears on the cooling curve. The reason is analyzed: after the molten iron enters the crucible, it begins to solidify and crystallize. The Eutectic Transformation of gray cast iron will release the latent heat of crystallization, which will increase the temperature of gray cast iron. After the latent heat of crystallization is released, the temperature will drop again, as shown in the figure.
It can be seen from the table that with the addition of rare earth alloy, the eutectic crystallization time first increases and then decreases, and it is the longest when the addition amount of rare earth alloy is 0.1%. The reaction between rare earth and oxygen and sulfur in molten iron to form rare earth oxide and rare earth sulfide is an exothermic process, which can increase the latent heat of crystallization, but with the increase of rare earth addition amount, The undercooling effect of rare earth and the chilling effect of molten iron increase and reduce the eutectic crystallization time. The increase of eutectic crystallization time is conducive to the precipitation of graphite, which shows that the addition of rare earth alloy can promote the graphitization of gray cast iron, which is consistent with the change of white mouth width introduced. Due to the undercooling effect of rare earth, the eutectic undercooling degree of gray cast iron increases, so as to reduce the graphite nucleation time and refine the grain.
| Rare earth addition (%) | 0.0 | 0.1 | 0.2 | 0.25 | 0.3 |
| Peak(°C) | 1343.8 | 1325.4 | 1369.8 | 1350.4 | 1370.0 |
| Ts(°C) | 1254.8 | 1253.3 | 1232.3 | 1237.3 | 1234.8 |
| Tsr(°C) | 1142.2 | 1122.6 | 1142.9 | 1123.2 | 1130.9 |
| Scope of eutectic problem | 112.6 | 130.7 | 89.4 | 114.1 | 103.9 |