The chemical composition of high chromium cast iron in the test is shown in the table, and the analysis of relevant alloy elements.
| C | Si | Mn | B | Cu | Cr | W | Mo | Ni | |
| HCCI | 3 | <0.8 | 0.9 | 0.3 | 2.5 | 22 | 0.5 | 0.5 | 0.5 |
1.Main phase elements C and Cr
The chemical composition of high chromium cast iron can determine its microstructure and mechanical properties. The influence of chemical composition on the mass percentage, type, shape and distribution of carbides should be considered in the design of relevant composition. The quantity and morphology of carbides in high chromium cast iron are determined by the mass percentage of elements C and Cr. in addition, the content of elements C and CR also has a very important influence on the matrix structure.
With the increase of chromium content, the carbide structure type gradually changes from the original network cementite m3c to the isolated M7C3. The hardness of carbides has also been greatly improved. The original m3c hardness range is 840-1100hv, while the hardness range of M7C3 after transformation is 1300-1500hv. Therefore, the eutectic carbide M7C3 is the most important anti-wear phase in the structure of high chromium cast iron. Chromium and carbonization can improve hardness, improve wear resistance and reduce free graphite. They are important modifying elements. Especially in the wet grinding environment, the solid solution of chromium in austenite can improve the corrosion and oxidation resistance of cast iron.
On the other hand, the addition of excessive chromium will lead to the production of excessive residual austenite and M23C6 carbides, which will reduce the wear resistance of the material. For carbon element, the carbide volume fraction and hardness of cast iron are in direct proportion to it, and the wear resistance of the material is also improved. However, the addition of excessive carbon will reduce the impact toughness of cast iron, which should be considered in the design of material composition.
According to the analysis, the high hard isolated strip carbide M7C3 must be formed in the structure of high chromium cast iron. At this time, the Cr content should be greater than 12% and the cr/c should be greater than 3.5. From the perspective of heat treatment processability of high chromium cast iron, in order to improve the material extraction and obtain high hardness martensite structure after heat treatment, the cr/c should generally be controlled to be higher than 7. From the perspective of controlling the volume fraction of material carbide, The content of carbide can determine the wear resistance of the material, and also has a certain impact on the toughness. In order to meet the service conditions of the wet ball mill, it is appropriate to control the amount of carbides at about 34%. It is considered that when the carbon mass fraction is 3%, the high chromium cast iron will obtain the best wear resistance. Therefore, the carbon mass fraction of the high chromium cast iron in the test is designed to be 3%, and the chromium mass fraction can be indirectly calculated by the formula.
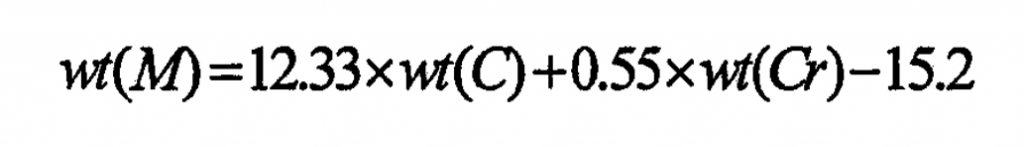
Where WT (m), WT (c) and WT (CR) respectively represent the mass fraction of carbide, carbon and chromium;
The mass fraction of Cr and C in the high chromium cast iron used in the test is 22% and 3% respectively.
2.Moisture and corrosion resistant elements B and Cu
In the same abrasive material environment, the wear of the inner liner of the wet ball mill is much more serious than that of the dry ball mill. Take the familiar knife sharpening as an example. The wet knife sharpening with watering is much more efficient than the dry knife sharpening. If the conductive medium (salt, acid and alkali) is added to the water, the knife sharpening speed will be further accelerated. The mechanism can be explained as follows: most metal materials are composed of two or more phases, and the polarity and potential of each phase in the tissue are different, When the potential difference between different phases is placed in the conductive medium (salt, acid, alkali), a loose electrochemical corrosion layer is formed on the surface of metal materials. Especially in the running ball mill, the scraping of materials and grinding balls will quickly remove it. In this cycle, wear and corrosion interact, resulting in rapid failure of wear-resistant materials. It can be seen that the potential of carbides in high chromium cast iron is much higher than that in the matrix structure. The electrochemical corrosion makes the martensite matrix structure loose, and even the hard carbides have no “base”. Therefore, the service life of high chromium cast iron lining plate in the wet ball mill is very limited.
In view of the mechanism of wet wear, elements were added to the material to increase the potential of matrix structure and reduce the potential difference between carbide and high chromium cast iron, so as to improve the electrochemical corrosion resistance of high chromium cast iron. In addition, at that time, precipitation strengthening can be produced.
The addition of boron (b) to cast iron can significantly improve its wet abrasion resistance and extraction permeability. Boron is a very cost-effective element with strong boron activity and easy to combine with oxygen. It is necessary to strictly control the material source and smelting process (such as adding deoxidation and argon blowing refining process outside the furnace) to stabilize the boron yield. In addition, boron can improve the stability of undercooled austenite, and has a stronger effect on improving the extraction permeability of cast iron than chromium, molybdenum, nickel and other alloy elements.
