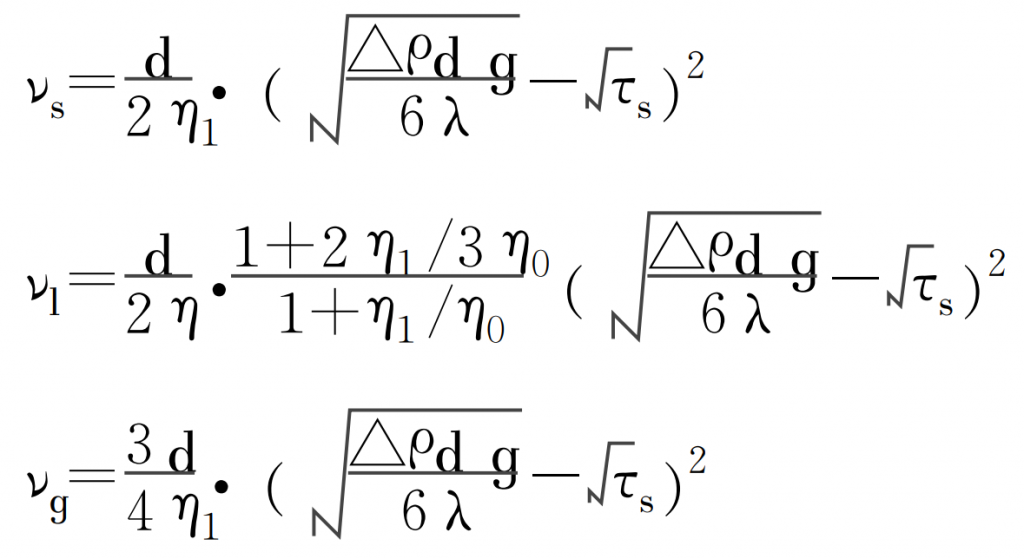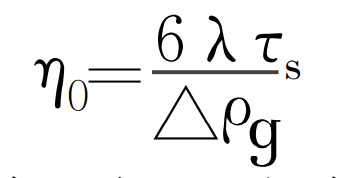It can be seen from the chemical composition analysis, microstructure metallography and SEM observation results of the part alloy that the overall chemical composition of the part basically meets the standard requirements, and the microstructure of the core of the test bar basically meets the design requirements. There are serious pitting defects on the surface of the test bar. The cavity within about 5mm below the surface is called subcutaneous pores, which are involved in Ca, Si, Cl, s and other inclusion elements. The existence of these elements is the possible cause of pitting corrosion of parts. For halogen elements such as Cl -, F -, etc., the atomic radius is relatively small (the crystallization radius of Cl – is 1 ∙ 81pm and the atomic radius of F – is 0 ∙ 072nm), so it has strong penetration and is easy to break through the oxide film and produce pitting corrosion. Therefore, al is very easy to corrode in the solution containing halogen ions. Studies have shown that when aluminum is quenched, for example, the quenching medium water contains (50 ~ 100) × At 10-6cl -, pitting corrosion will occur on the surface of aluminum alloy during quenching. If Cl – has been attached to the part surface before quenching, a large Cl – concentration may be formed on the part surface with Cl – in quenching water, and pitting corrosion is natural. Although the atomic radius of sulfur on the surface of parts is larger than that of Cl – and F -, if sulfur exists in the form of acid, acidic solution will be formed in the micro area on the surface of parts in humid environment. The results show that when the pH value of the micro region is greater than a certain value, the passivation film cannot be formed on the surface of aluminum alloy, and aluminum will dissolve rapidly.
Looking at the metallographic and SEM morphology of the subcutaneous pores and the energy spectrum analysis results of the components contained in the inner wall of the pores, it can be seen that the subcutaneous pores are formed during the solidification of metal liquid. The formation of such pores is controlled by a typical heterogeneous nucleation mechanism. If there are bubbles and inclusions in the metal during melting or casting, the stable floating speed of bubbles and inclusions is:
Where:
ν S-velocity of solid particle
ν L – velocity of particles in liquid phase
ν G – velocity of particles in gas phase
D – particle diameter
Δρ- Density difference between particle and alloy
λ- constant
η- Viscosity of liquid particle
τ S-yield limit of alloy
η 0 – viscosity of alloy liquid during solidification
η 1 – viscosity coefficient of liquid particle
G – gravitational acceleration.
According to the formula, the floating velocity of heterogeneous particles is mainly affected by the size and morphology of particles and the viscosity of alloy solution. The viscosity of alloy liquid depends on the characteristics of alloy itself and the superheat of alloy liquid. By substituting the condition that the floating velocity of heterogeneous particles is zero into the formula, the critical condition for the floating and sinking of heterogeneous particles can be obtained:
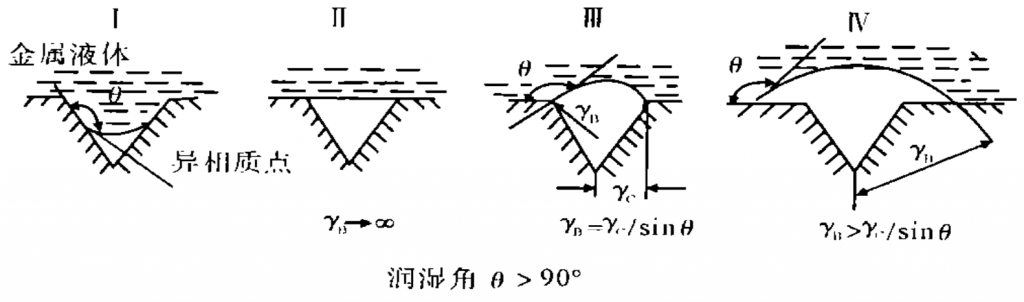
When the temperature is constant, if the inclusion size is less than the critical size, the inclusion will stay in the casting to form metallurgical inclusion. Metallurgical inclusions are conducive to the formation of subcutaneous pores. The figure is the schematic diagram of heterogeneous particle nucleation mechanism of subcutaneous stomata.
If the alloy has heterogeneous particles (inclusions, irregular shapes) as shown in the figure during melting and casting, if gas is involved in the groove, there is a wetting angle between the liquid and the groove of the inclusion due to the action of surface tension. During the solidification process of the alloy, due to the change of the alloy from liquid to solid, it will shrink greatly and the radius of the gas core will continue to increase, Finally, as shown in Figure IV, subcutaneous pores are formed. Inclusions containing elements such as s and CL were detected in the subcutaneous pores of the studied parts, which means that the heterogeneous phase in the alloy is a compound containing s and CL. Moreover, the size of this compound may be relatively small, and it does not float to the surface of liquid metal during alloy refining and is removed as slag.