Crystal growth is a process in which the atoms in the liquid phase migrate and stack to the surface of the crystal, thus making the solid-liquid interface face the liquid. Therefore, the growth mechanism of the crystal depends on the S / L interface structure, which has a great influence on the final morphology of the crystal. Under the action of pressure, the migration of atoms is affected, and the thermodynamic and kinetic conditions of crystal growth are also changed, which further affects the growth mode of crystal.
To discuss the growth characteristics of carbides, it is necessary to understand Jackson factor. When Jackson factor α When the free energy is less than or equal to 2, 50% of the atoms are deposited on the interface, which is called rough interface (atomic scale). Most metals fall into this category. When Jackson factor α> At 5, there are two kinds of atomic positions at the interface, that is, there is little atomic deposition or the interface is not occupied by atoms, the solid-liquid interface is smooth (atomic scale), and nonmetallic and some organic compounds belong to this structure. When α= In the range of 2 ~ 5, the deposition of interface atoms is complex, which combines rough and smooth forms and can transform under certain conditions.
The carbides in chromium white cast iron belong to intermetallic compounds. The Jackson factor of carbides is calculated α It is between 2 and 5, that is, in the range shown in Figure 1. The Jackson factor of different types of carbides is obtained by calculation α Fe7C < α( Fe,Cr)3C < α( Fe,Cr)7C3。
It has been mentioned that the influence of pressure on the melting temperature of the alloy is shown in the formula:
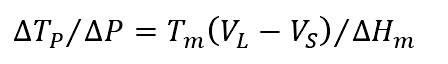
The influence of pressure on Jackson factor can be described as follows:
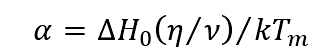
According to the formula, the melting temperature Tm increases when the pressure increases, which is substituted into the formula to calculate the Jackson factor α Lower. The results show that the pressure increases the roughness of the solid-liquid interface, and even the carbides grown in small plane (smooth interface) will transform into non small plane (rough interface) growth under higher pressure. The transformation of the front edge of the solid-liquid interface is shown in Fig. 2
Taking the alloy in the experiment as an example, the carbides in the solidification structure are m3c and M7C3. When the Jackson factor of M7C3 is larger than that of m3c, the difficulty of reducing Jackson factor will be different. That is to say, m3c carbides are more susceptible to the effect of pressure, so that the growth mode of facet is changed to non facet. The Jackson of m3c is relatively small and the growth rate is relatively fast. During the growth process, there may be “crazy growth”, which will lead to the interconnection of carbides and the formation of network structure. When the austenite reaches the nucleation condition, the continuous growth space of austenite will be limited by the network carbide.


