Carbon content in bovine eye ferrite
The microstructure of as cast ductile iron with MS = 0.25cm consists of 73.7% pearlite and 26.3% bovine eye ferrite. The carbon content in bovine eye ferrite was analyzed by EPMA. The measuring point was located at the center of bovine eye ferrite, about 3.5 μ m away from graphite ball and pearlite respectively, as shown in Fig. 3-3. The carbon contents in the ferrite are 0.658%, 0.447% and 0.527%, respectively, with an average value of 0.54%. This indicates that under casting conditions, more carbon atoms will be dissolved in the ferrite by rapid cooling.

Carbon content in bovine eye ferrite
The microstructure of as cast ductile iron with MS = 0.25cm consists of 73.7% pearlite and 26.3% bovine eye ferrite. The carbon content in bovine eye ferrite was analyzed by EPMA. The measuring point was located at the center of bovine eye ferrite, about 3.5 μ m away from graphite ball and pearlite respectively, as shown in Fig. 3. The carbon contents in the ferrite are 0.658%, 0.447% and 0.527%, respectively, with an average value of 0.54%. This indicates that under casting conditions, more carbon atoms will be dissolved in the ferrite by rapid cooling. In the process of graphite ball growth, due to the diffusion of carbon atoms, a “carbon escape zone” will be formed around the graphite ball; when it grows to a certain size, it will be surrounded by austenite shell (halo); further growth of graphite ball needs to transport (diffuse) carbon atoms through austenite shell. The theory shows that the carbon concentration in the molten iron around the graphite ball increases with the distance from the graphite ball before the formation of the halo (austenite shell).

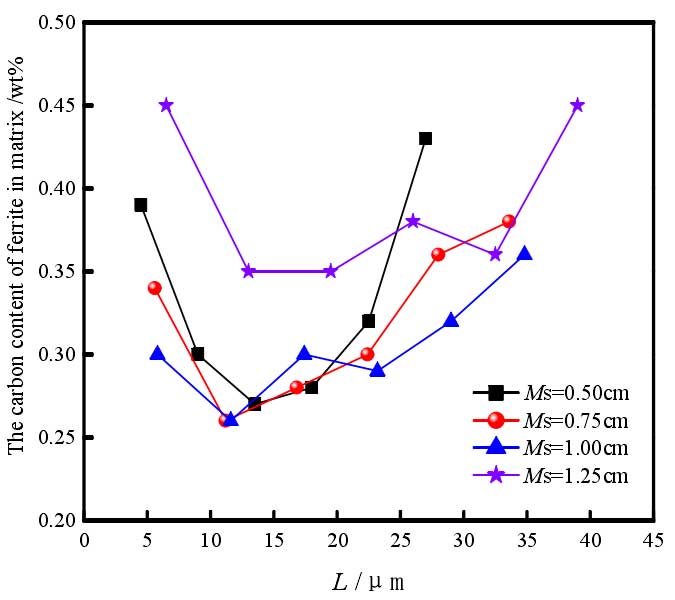
On the other hand, in the process of halo formation and thickening, austenite begins to decompose into ferrite, and ferrite nucleates preferentially at the graphite / austenite interface, and grows and thickens along the normal direction of graphite sphere. The austenite side of the ferrite / austenite interface is a high carbon region, and carbon atoms generally diffuse to the surface of graphite ball in the opposite direction to the growth direction of ferrite. Obviously, in the path of carbon diffusion, the spherical area of ferrite spherical crystal containing graphite balls is shrinking, which inevitably leads to the rapid increase of carbon atom concentration in the shell, thus forming a gradient distribution of carbon concentration in the ferrite around the graphite ball. Then, between the two graphite balls, it also forms a “U” type distribution characteristics. Moreover, due to the significant difference in diffusion coefficient and solubility of carbon atoms in molten iron and solid iron, the concentration distribution of carbon around the graphite ball is different in different states. When the casting modulus is small (MS = 0.50cm) and large (MS = 1.25cm), the carbon content in ferrite is higher, with the average value of 0.33% and 0.39%, respectively. This is mainly related to the diffusion kinetics of carbon atoms. The former has faster cooling rate and slower diffusion rate of carbon atoms; the latter has less graphite balls and longer diffusion distance of carbon atoms, both of which make more carbon atoms remain in ferrite and increase the solid solubility of ferrite carbon.
