The solidification process can be regarded as a binary system with solid-liquid two-phase equilibrium. The degree of freedom of the system is 2 according to the phase rule. That is to say, among the three variables of temperature (T), pressure (P) and concentration (c), the concentration (i.e. phase point) is controlled by temperature and pressure. When the temperature and pressure are constant, the phase point and equilibrium condition of the system can be determined. Therefore, the changes of temperature and pressure have an important influence on the solid-liquid interface equilibrium during solidification. In addition, these changes will also affect the growth rate and stability of the interface, and ultimately affect the phase transformation and microstructure composition of the solidification structure.
(1) The relationship between pressure and nucleation rate can be described as follows
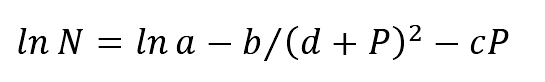
Where:
N — nucleation rate;
P — pressure value (PA);
a. B, C — constant coefficient;
The curve shown in Fig. 1 can be obtained from the above equation.
At constant temperature, the nucleation rate first increases and then decreases with the increase of pressure. In the process of industrial squeeze casting with the pressure range of 80 ~ 100 MPa, increasing the pressure can significantly improve the nucleation rate of grain and have a significant effect on grain refinement.
(2) The relationship between pressure and melting entropy can be described as follows
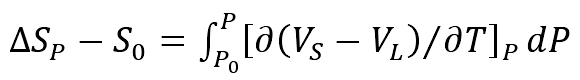
Where:
Δ S0 — melting entropy of alloy at atmospheric pressure (J / molk);
Δ SP — melting entropy under pressure P (J / molk);
VL — molar volume of liquid phase (m) ³/ mol);
Vs — molar volume of solid phase (m) ³/ mol)。
The pressure increases the melting entropy of the alloy, the irregular thermal motion of the atom becomes active, and the disorder degree of the system increases.
(3) The relationship between pressure and solute equilibrium partition coefficient can be described as follows
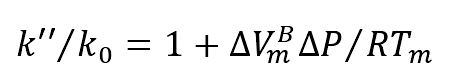
Where:
K “- when the system pressure changes Δ The equilibrium distribution coefficient of solute at p;
Δ𝑉𝐵 m — the change of molar volume of solute component B from liquid to solid;
It can be concluded from the above formula that the solute partition coefficient increases with the increase of pressure.
(4) The relationship between pressure and melt viscosity can be described as follows
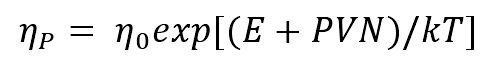
Where
η 0 — viscosity of melt under normal pressure (pa.s.);
E — activation energy of liquid alloy (kJ / mol);
η P — viscosity of alloy melt under high pressure (pa.s.);
K — Boltzmann constant (J / k);
V — volume of liquid alloy (M3);
N——6.02 × 1023;
T — temperature (k).
The calculation shows that the viscosity of liquid metal increases with the increase of pressure.
(5) The relationship between pressure and equilibrium melting point can be described as follows
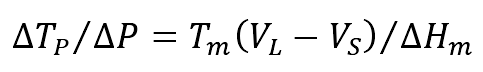
Where
Δ P — pressure change value (PA);
Δ T — corresponding change Δ The melting temperature difference (k) of P value;
TM — melting temperature (k);
Vs, VL – LKG solid and liquid volume (M3);
HM — latent heat of alloy melting (kJ / kg).
The above equation is Clausius Clapeyron equation. The volume of common cast iron increases during melting, that is, when vl-vs > 0, so the melting temperature of alloy increases with the increase of pressure.
(6) Effect of pressure on equilibrium phase diagram of alloy
In the process of pressure solidification, the pressure has an important influence on the physical properties, phase equilibrium, crystallization process and microstructure of metals. The melting point of cast iron alloy changes under pressure, which is one aspect of pressure changing alloy phase diagram. A large number of experiments and theoretical calculations show that when the pressure increases to a certain value, the phase diagram of the alloy will change greatly. Such as changing the position of phase transition point, changing the area of different phase area, changing the properties of known phase, forming new phase or new phase area, etc.
The pressure can change the phase diagram of Fe-C graphite and Fe-Fe3C, make the eutectic point of Fe-C graphite move to the direction of low temperature and rich C, and increase the temperature of Fe-Fe3C eutectic point. In the temperature range of 1473-2073 K, a series of melting experiments were carried out in the Fe-C system with pressure up to 25 GPa. The relationship between eutectic temperature, composition and pressure, the distribution of C in solid-liquid phase and the change of melting relationship of iron-bearing carbide were determined, as shown in Fig. 2
As a very promising special smelting method, pressure metallurgy technology has an important influence on metallurgical reaction and solidification process. The increase of pressure will change the direction of metallurgical reaction, Gibbs free energy and reaction rate, and some impossible reactions will be carried out in the atmosphere. Some beneficial effects of pressure on metal solidification are obtained, including accelerating the cooling rate, increasing the solidification nucleation rate, reducing the critical nuclear radius, refining the macro / micro structure and eliminating the defects under high stress solidification.
In the study of alloy solidification process, the existing research does not pay special attention to the influence of pressure, because most of the knowledge is established under atmospheric pressure, and temperature is almost the only variable to regulate and control the solidification process. However, the effect of pressure must be fully considered when preparing amorphous materials, quasicrystals and nanocrystals.


