Due to its high strength, high plasticity, as well as good low-temperature impact toughness and weldability, copper containing cast steel materials have been widely used in the manufacturing of new ship or offshore platform components in recent years. The maximum wall thickness of the large copper containing cast steel manufactured by the steel casting manufacturer is 300 mm. During mechanical performance testing after heat treatment, abnormal fracture occurred. The strength index of the cast steel meets the technical requirements, but the plasticity index is lower than the technical requirements. In order to identify the reasons for the non conformity of plasticity indicators, ZHY Casting analyzed the residual mechanical performance test samples of the steel casting and determined the reason for the decrease in plasticity.
1.Test materials
The copper containing steel casting was smelted in an electric arc furnace and refined by LF+VD before casting (see Table 1). Due to the high content of alloys such as Mn, Ni, Cr, Cu, etc., this steel grade is sensitive to gases, especially hydrogen content. To reduce the gas content in the molten steel, argon gas protection is used during the pouring process.
| C | Si | Mn | P | S | Ni | Cr | V | Cu |
| ≤0.13 | ≤0.50 | ≤0.80 | ≤0.020 | ≤0.030 | ≤1.60 | ≤0.30 | ≤0.10 | ≤0.80 |
After the steel castings are poured, insulated, and sandblasted, the water riser is first cut and then subjected to heat treatment. The heat treatment process parameters are selected according to the recommended parameters in the process documents, and the insulation time is determined based on the maximum wall thickness of copper containing steel castings (see Table 2).
| Process | Heating temperature (℃) | Insulation time (h) | Cooling method |
| Preprocessing | 640 ± 10 | 48 | Furnace cooling |
| Normalizing | 920 ± 10 | 10 | Air cooling |
| Tempering | 620 ± 10 | 7 | Air cooling |
After heat treatment of copper containing cast steel parts, standard tensile specimens with a diameter of Ø 10 mm and a set of standard Charpy V-shaped impact specimens are taken from their performance testing blocks for testing. The tensile test is carried out in accordance with GB/T 228, and the impact test is carried out in accordance with GB/T 229 (see Table 3). According to Table 3, the yield strength, tensile strength, and impact toughness of the copper containing cast steel meet the technical requirements, but the elongation after fracture index is lower than the technical requirements.
| Project | ReL(MPa) | Rm(MPa) | A(%) | Z(%) | -40 ℃ KV2(J) | -Average value of KV2 at 40 ℃ (J) |
| Required value | ≥370 | ≥490 | ≥20 | ≥40 | ≥27 | ≥ 27 test values |
| Experimental value | 406 | 526 | 18.0 | 57 | 45 43 55 | 47 |
2. Experimental analysis
To analyze the reasons for the inadequate elongation after fracture of copper containing cast steel parts, scanning electron microscopy fracture analysis, metallographic structure analysis, and finished product chemical analysis were performed using tensile and impact residual samples.
2.1 Fracture analysis
Cut the tensile residual sample to an appropriate height, clean the fracture surface with ultrasonic waves, and observe it under the FEI Quanta400 electron scanning microscope (see Figure 1). It was found that the necking of the tensile fracture surface was small, and there were no typical plastic material necking, shear lips, and other characteristic morphologies. There were many nearly circular or circular pits on the fracture surface (see Figure 1 (a)), and the pits were relatively smooth quasi solution inner sections, without ductile dimples, The core of the pit has loose defects, and the cross-section inside the pit is radial. The central area is loose, and the surface of the loose defect area is smooth and in a free surface state (see Figure 1 (b)~(d)).
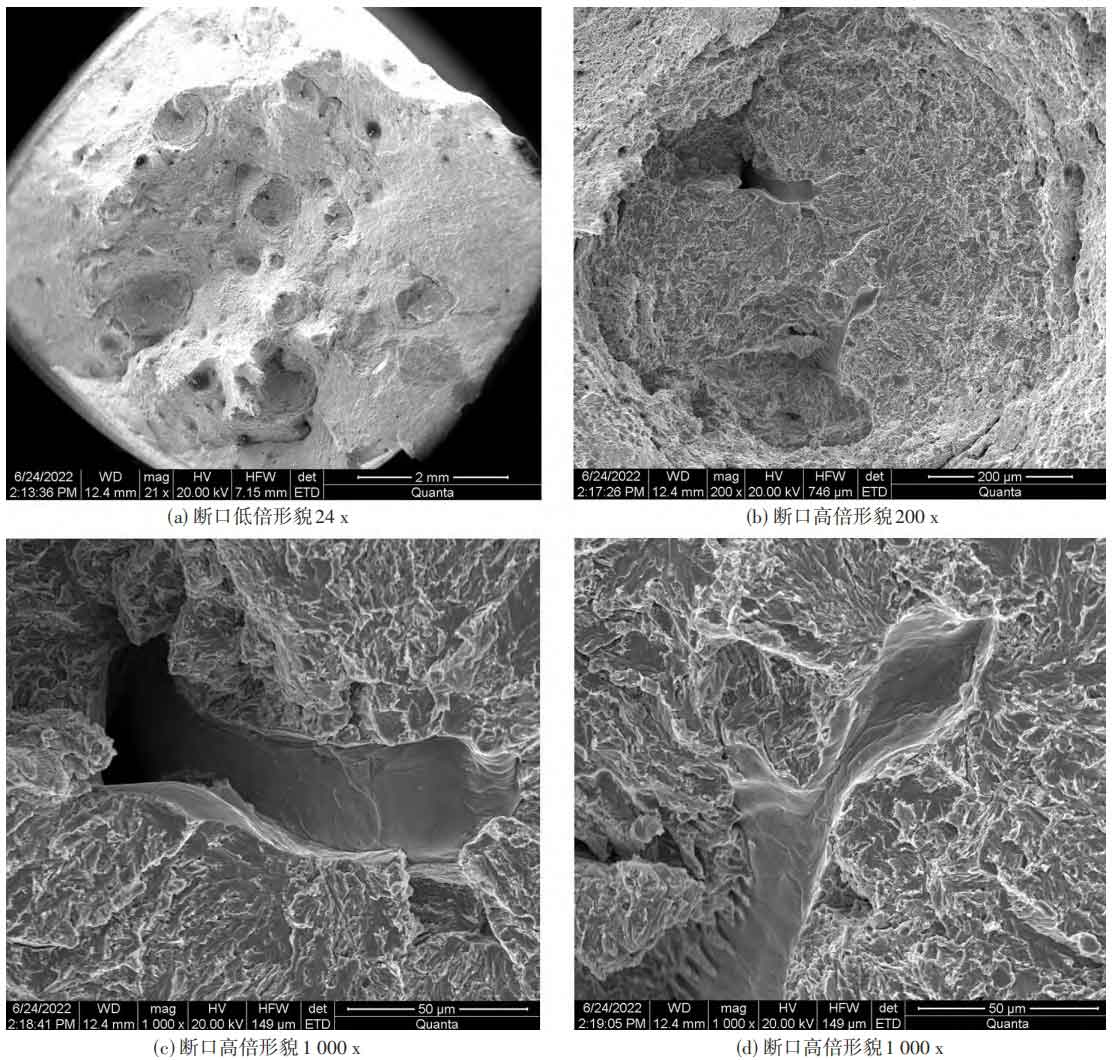
2.2 Metallographic analysis
Observing the impact residual samples after polishing, polishing, and corrosion using Axio observer 5m optical microscope, it was found that the microstructure after heat treatment is ferrite+pearlite+a small amount of bainite tempered structure (see Figure 2), with relatively small grain size and obvious microsegregation areas. Pearlite and bainite areas are distributed around ferrite dendrites.
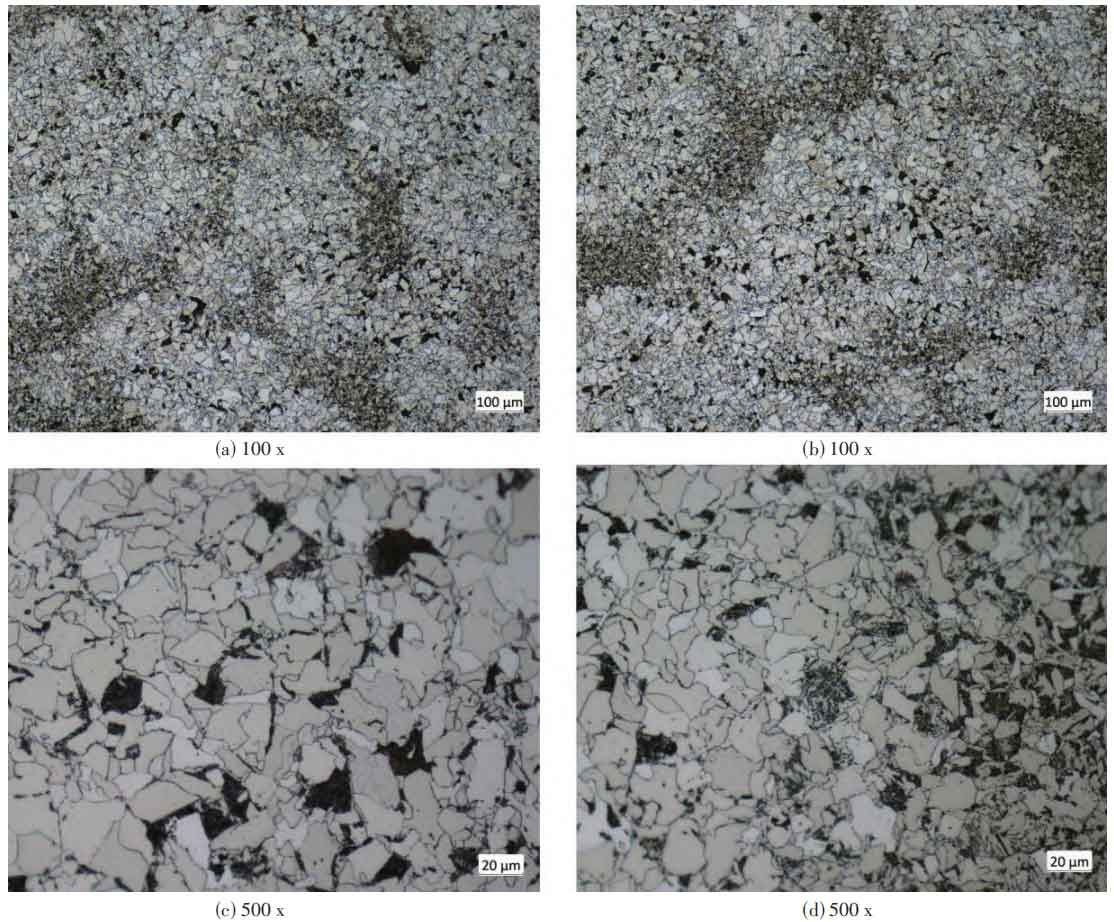
2.3 Chemical analysis
Samples of tensile and impact residues were taken and subjected to chemical analysis of the finished product using a CS600 carbon sulfur analyzer and an ICAP6500 plasma spectrometer (see Table 4). All element contents met the material technical requirements. The RHEN602 hydrogen detector was used to detect the hydrogen content, and the result was 5.5 ppm. However, the hydrogen content in the molten steel analysis report of copper containing steel castings was only 1.5 ppm, indicating an abnormal hydrogen content in the finished product.
| Project | C | Si | Mn | P | S | Ni | Cr | V | Cu |
| Requirement | ≤0.13 | ≤0.50 | ≤0.80 | ≤0.020 | ≤0.030 | ≤1.60 | ≤0.30 | ≤0.10 | ≤0.80 |
| Actual measurement | 0.11 | 0.18 | 0.76 | 0.008 | 0.005 | 1.45 | 0.26 | 0.002 | 0.70 |
3. Cause analysis and discussion
According to the test results, it can be seen that the hydrogen content in copper containing steel castings is relatively high, and there are a large number of micro void defects inside the copper containing steel castings. During the cooling process of heat treatment, as austenite decomposes and the temperature decreases, the solubility of hydrogen in steel decreases. Hydrogen atoms precipitate from the steel, diffuse to some micro voids inside the copper containing cast steel, and form molecular states, generating considerable pressure. During the tensile test, these micro voids become weak areas, and under the combined action of external stress and hydrogen induced internal stress, brittle failure first occurs, resulting in unqualified elongation of the tensile specimen after fracture.
Possible reasons for abnormal increase in hydrogen content:
(1) Smelting adopts LF+VD, vacuum refining can ensure lower hydrogen content, and there are inspection reports to support it, which can be ruled out.
(2) Although argon gas protection is used during pouring, it was found through records that the detachment of the argon gas tube during pouring can lead to an increase in hydrogen content.
(3) Before pouring, the cavity is filled with argon gas for protection, preventing hydrogen from entering the molten steel in the cavity air, and the effect is difficult to determine.
(4) If the sand mold is baked unevenly and contains moisture, it will add hydrogen to the copper containing cast steel products. After investigation, it was found that there may be insufficient baking time due to cloudy and rainy weather during the process of beating and laying cores.
4. Improvement measures and hydrogen expansion calculation
The micro porosity defects inside large copper containing steel castings are difficult to eliminate and avoid. Only by increasing the dehydrogenation heat treatment insulation time can the hydrogen content in copper containing steel castings be reduced, and the performance of copper containing steel castings be improved. Therefore, the author adjusted the dehydrogenation time to 72 hours, 144 hours, and 216 hours.
Calculate the hydrogen expansion amount for different hydrogen removal times based on the standard equation of hydrogen expansion effect for large forgings:
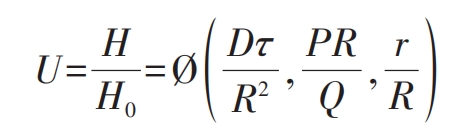
In the formula:
U – concentration standard of hydrogen in forgings;
H0-H0 Original hydrogen content in forgings before dehydrogenation annealing;
H – hydrogen content in forgings after dehydrogenation annealing;
D τ/ R ^ 2- The time standard required to reach the concentration standard U, commonly known as the Freund’s standard, expressed as F0;
D – Hydrogen diffusion coefficient, can be obtained by looking up the table;
τ— Diffusion time (h);
R – radius of cylindrical forging (cm);
PR/Q – Bi standard number, usually expressed as Bi. When calculating the Bi standard number, the ratio P/Q is approximately taken as 1/(2.5 cm-1);
P – Permeability coefficient;
Q – Permeability coefficient;
R/R – positional accuracy, for forgings below 300 mm, the positional accuracy is taken as 0;
R – Calculate the radius of the position.
Based on the above methods, obtain the Freund’s number F0, Bi, and position number r/R for dehydrogenation times of 72 h, 144 h, and 216 h, and then look up the table to obtain the concentration number U. Calculate the remaining hydrogen content H after different dehydrogenation times (see Table 5).
| Dehydrogenation time (h) | Fukushima number F0 | Bi’s exact number | Position accuracy r/R | Concentration standard U | Remaining hydrogen content H |
| 72 | 0.247 52 | 6 | 0 | 0.546 6 | 3.0 |
| 144 | 0.495 04 | 6 | 0 | 0.191 8 | 1.0 |
| 216 | 0.742 56 | 6 | 0 | 0.073 1 | 0.4 |
According to the limit hydrogen content without white spots of commonly used steel grades, it can be inferred that the copper containing cast steel has a limit hydrogen content without white spots of ≤ 1.8 ppm. The insulation time after the improvement of the dehydrogenation heat treatment process is 144 hours.
5. Verification test
After 144 hours of dehydrogenation heat treatment and positive tempering performance heat treatment, samples were taken again for mechanical performance testing. The plasticity and toughness indicators of the product were improved, with the elongation after fracture increased from 18.0% to 25.0%, the cross-sectional shrinkage increased from 57% to 70%, and the impact toughness also improved (see Table 6).
| Project | ReL(MPa) | Rm(MPa) | A(%) | Z(%) | -40 ℃ KV2(J) | Average value of KV2 at -40 ℃ (J) |
| Required value | ≥370 | ≥490 | ≥20 | ≥40 | ≥27 | ≥ 27 test values |
| Dehydrogenation for 48 hours | 406 | 526 | 18.0 | 57 | 45 43 55 | 47 |
| Dehydrogenation for 144 hours | 410 | 538 | 25.0 | 70 | 64 78 74 | 72 |
The RHEN602 hydrogen analyzer was used for hydrogen content detection again, and the result was 0.5 ppm, indicating a good hydrogen expansion effect.
6. Conclusion
By conducting macroscopic analysis, microscopic analysis, metallographic analysis, and chemical composition analysis on the fracture surface of the sample, the following conclusions were obtained:
(1) The high hydrogen content and microscopic defects inside copper containing steel castings are the reasons for the unqualified plasticity of copper containing steel castings.
(2) The microstructure of copper containing steel castings after heat treatment is ferrite+pearlite+a small amount of tempered bainite.
(3) To prevent excessive hydrogen content in molten steel, large copper containing steel castings should be subjected to vacuum treatment during smelting, and argon protection should be taken during pouring. Sand molds should be fully baked to prevent hydrogen from entering the surrounding environment.
(4) After long-term hydrogen expansion treatment, the hydrogen content in copper containing cast steel parts is significantly reduced, and the plasticity and toughness indicators of the products are improved.
