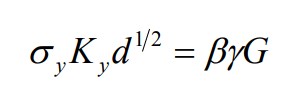A large number of experimental studies have proved that in general, the higher the purity of metal, the better its ductility. The addition of alloying elements can promote the transformation of fracture mode from ductile fracture to brittle fracture, and the addition of alloying elements can strengthen the fatigue fracture of materials. The strengthening mechanism is mainly divided into the following types:
Strengthening lattice: the addition of alloy elements strengthens the material lattice, and the ability to resist stress deformation is also enhanced after lattice strengthening, which makes deformation difficult and difficult to produce stress concentration, improves the stress level required for fatigue crack nucleation and initiation, prolongs the nucleation time of crack, and increases the energy required for crack propagation after lattice strengthening, The effective energy of material fracture is increased and the crack propagation is hindered. Therefore, the addition of alloy elements plays a role in resisting the nucleation, initiation and propagation of fatigue cracks, and effectively improves the fatigue properties of the material.
Change of microstructure: the addition of some alloy elements can promote grain refinement and improve the material strength. For example, the tic formed by adding a small amount of Ti to V alloy can weaken the trend of crack development along the grain boundary. However, sometimes alloy elements are easy to gather at the grain boundary, produce segregation, form stress concentration and intergranular fracture. Of course, there are opposite phenomena. For example, adding Mn to steel can eliminate the grain boundary segregation of Sn.
Stress corrosion, hydrogen embrittlement, nitrogen embrittlement and liquid metal embrittlement: when the alloy elements that seriously reduce the stacking fault energy are dissolved in the metal, such as Zn in Cu, the dislocations in Zn increase obviously; Then placing Zn in brittle environment will produce serious transgranular stress corrosion cracking along {111} plane. After the liquid metal contacts with the alloy, the interatomic bonding force on the grain boundary is weakened, the crack is easy to expand along the grain boundary, and the mixed fracture is transformed into pure intergranular brittle fracture.
Regardless of any crystal structure alloy, the main causes of brittle fracture are: the sharp increase of yield stress or work hardening rate, the accumulation of alloy elements on various internal and external surfaces, the difficulty of cross slip or the increase of mechanical twinning. According to Cottrell Petch formula, the effect of alloy elements on fracture can be known:
among σ Y is yield stress; KY is Petch slope; D is the grain diameter; When the deformation is torsion β ≈ 2, when stretching β ≈ 1, when tensile with notch β ≈1/3; γ Is the effective surface energy; G is the shear modulus of the material.