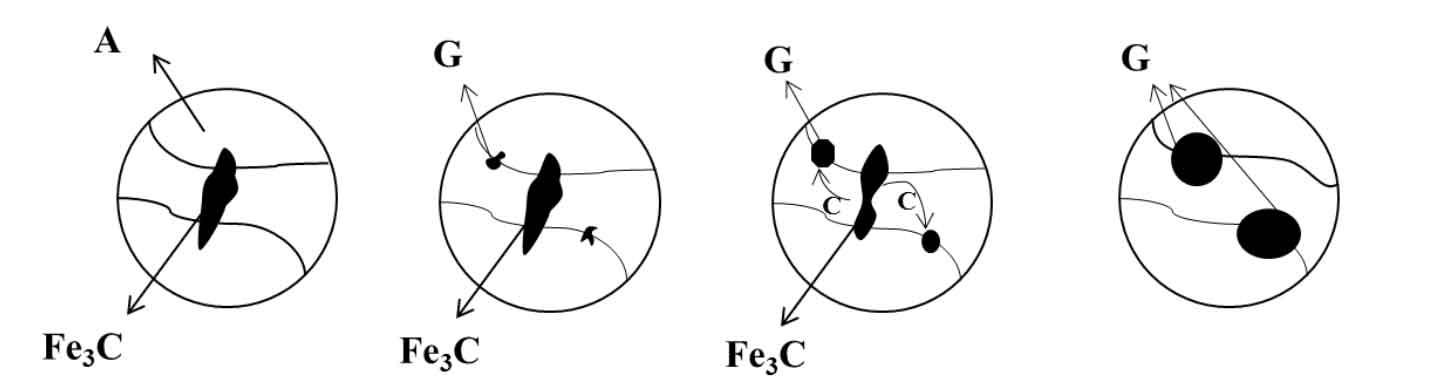
The solid-state graphitization process of cast iron is essentially a process of transformation from metastable cementite to stable graphite. Graphitization annealing process is shown in Figure 1. In the process of slow heating of gray cast iron, cementite gradually decomposes, and carbon atoms precipitate from cementite and form nuclei at the austenite interface. With the increase of heating temperature, carbon atoms continue to move from cementite to graphite to grow graphite. In the process of graphitization annealing, the decomposition ability of cementite and graphitization annealing temperature play a decisive role in the formation of graphite.
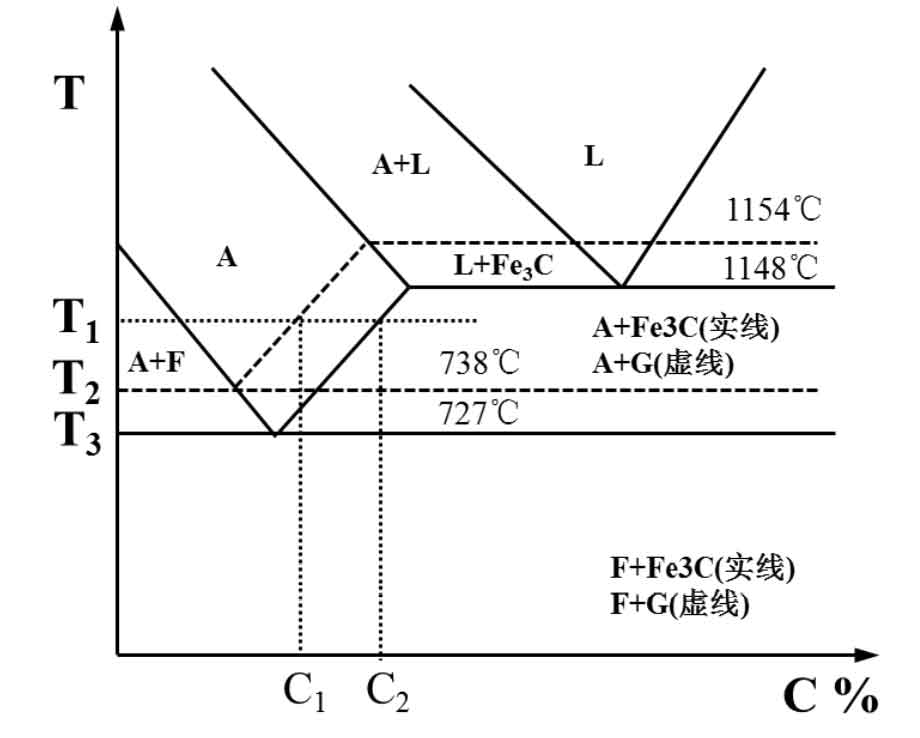
The graphitization annealing temperature of gray cast iron occurs in a wide temperature range, in which different temperatures correspond to the stable equilibrium of ferrite + austenite + graphite or the metastable equilibrium structure of ferrite + austenite + cementite. From the perspective of thermodynamics, the white cast iron structure and eutectic carbide in gray cast iron are extremely unstable under high temperature conditions. When the gray cast iron is heated above the eutectoid transformation temperature, the cementite in white cast iron is gradually decomposed into austenite and graphite, and the carbon content of austenite is changed at the same time. According to the iron carbon phase diagram (as shown in Figure 2), T2 and T3 are the eutectoid temperatures of cementite and ferrite transformation respectively. When the temperature rises to T1, the dissolved carbon element in austenite increases continuously, and the supersaturation amount of carbon element is between C1 and C2. If heat preservation is carried out at this temperature, carbon atoms will continue to diffuse and migrate from the “austenite cementite” interface to the “austenite graphite” interface, the precipitated carbon atoms will continue to dissolve into austenite, and the nucleated graphite will continue to grow at the same time. Therefore, as long as different heating temperature and holding time are controlled, different graphite morphology and matrix structure can be obtained after annealing.
During graphitization annealing of cast iron, the number of precipitated graphite cores and the diffusion ability of carbon atoms determine the speed of graphite formation. The more the number of precipitated cores of graphite in austenite, the shorter the diffusion distance of carbon atoms and the faster the rate of solid-state graphitization. Therefore, in order to speed up the graphitization annealing, the key is to speed up the decomposition of cementite and improve the diffusion ability of carbon atoms. In the process of graphite formation, carbon atoms first form nuclei on the austenite grain boundary or at the defects of the structure, and then the carbon atoms continue to diffuse and grow to the graphite core. At high temperature, the diffusion distance of carbon atoms is far, which makes the growth probability of graphite in all directions almost the same, so the graphite is in flocculent shape after annealing. Yin Yanna studied the effect of the amount of pearlite and graphite on the hardness and low-temperature impact toughness of nodular cast iron by holding the nodular cast iron with 2.3% carbon at 760 ℃ for 6 hours. After annealing, the pearlite structure in the matrix changed to full ferrite structure, and the amount of graphite increased. When the amount of graphite reached 400 pieces / mm2, The impact toughness reaches 29 J / cm2. Liu et al. Conducted high-temperature annealing experiments on white cast iron samples with poor toughness. The experimental results show that after holding at 1050 ℃ for 4 hours and then cooling to 720 ℃ for 10 hours, the white cast iron structure in the obtained samples is completely decomposed, the ferrite matrix structure becomes larger, and the apparent microhardness of the sample surface is reduced to 36 HRC after annealing at high temperature, However, the annealed white cast iron has better machinability. Ma Wenxu optimized the graphitization annealing process. After holding at 900 ℃ for 6 hours and cooling to 720 ℃ for 12 hours, the malleable cast iron with D-type graphite can be obtained. Compared with the traditional annealing process, the graphitization annealing time after the improved process is reduced by 40%. Zhang Fuquan and others conducted low-temperature graphitization annealing experiments on nodular cast iron. After annealing, the pearlite structure in nodular cast iron was transformed into ferrite, the impact toughness was greatly improved, and there were a few dimples in the impact fracture.