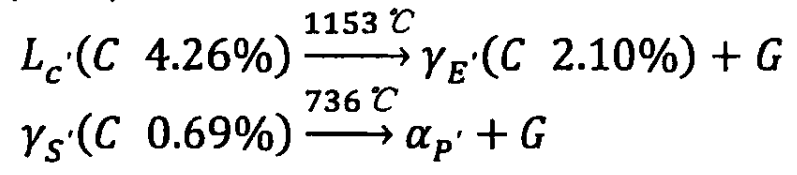Cast iron generally refers to multi-component iron carbon alloy with C content of 2.0% (wt%, the same below) W. Generally, the C content of industrial cast iron is 2.5-3.5%. C exists in gray cast iron in the form of W graphite and a small amount of W PA C. In addition to C, gray cast iron also contains 1-3% of Si, W, Mn, P, s and other elements. C. Si, Mn, s and P are the five basic elements in cast iron. In addition, according to the use and required properties of castings, cast iron also contains alloy elements such as Ni, Cr, Mo, A1, Cu, Sb and V.
Gray cast iron has high C content, and its matrix structure varies according to different cooling and solidification processes. It can be generally divided into three categories: ferrite matrix gray cast iron, pearlite ferrite matrix gray cast iron and pearlite matrix gray cast iron.
From the microstructure analysis of gray cast iron, the difference in classification is essentially the content of different forms of C in cast iron. Its main types are synthetic carbon (Fe3C) and G (graphite). When Fe3C is 0.8%, it is pearlite gray cast iron; When FESC is less than 0.8%, it belongs to pearlite ferrite gray cast iron; When C exists only in g state, it is ferritic gray cast iron.
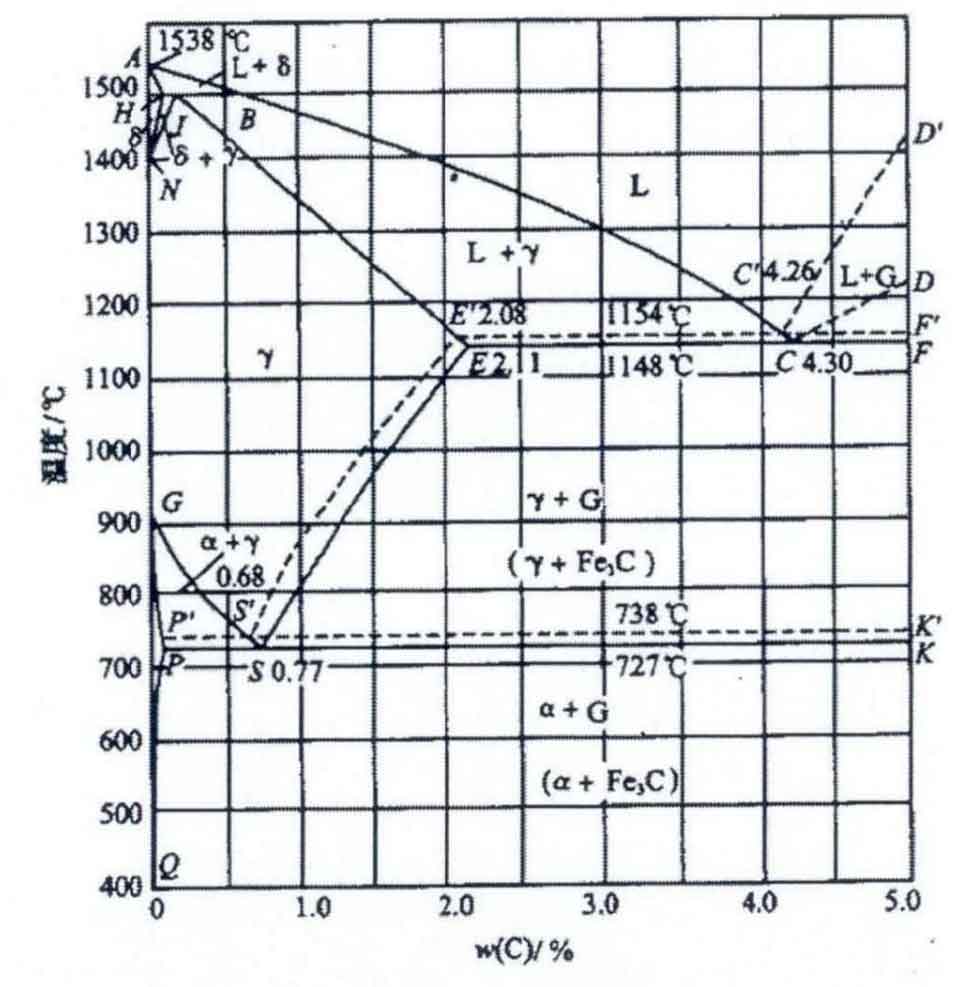
The basis of cast iron is inseparable from the iron carbon equilibrium phase diagram, which has theoretical guiding significance for the production of cast iron. When studying the crystallization process of gray cast iron, the iron carbon dual phase diagram is needed. As shown in Figure 1.2, W, the solid line represents the Fe-Fe3C phase diagram (white cast iron), and the dotted line represents the Fe-G phase diagram (white cast iron). Under suitable solidification conditions, graphite can be crystallized from Fe-C alloy with carbon content higher than 2.14%. For example, at a certain temperature, carburizing during heat treatment will decompose into austenite and graphite; Under the condition of holding temperature above and below eutectoid temperature or slow cooling, austenite in cast iron will be transformed into graphite and ferrite.
From the thermodynamic analysis, the free energy of Fe3C is relatively high and will decompose into austenite and graphite with low energy state under certain conditions. The transformation process of austenite plus Fe3C is balanced, so the Fe-Fe3C phase diagram is metastable and the Fe-G phase diagram is stable. From the perspective of crystallization kinetics, the formation of graphite in molten iron requires the aggregation of C and the outward diffusion of Fe. Compared with the formation of Fe3C (interstitial compound), its conditions require higher requirements.
The eutectic and eutectoid transformations in the Fe-C (graphite) phase diagram are as follows: