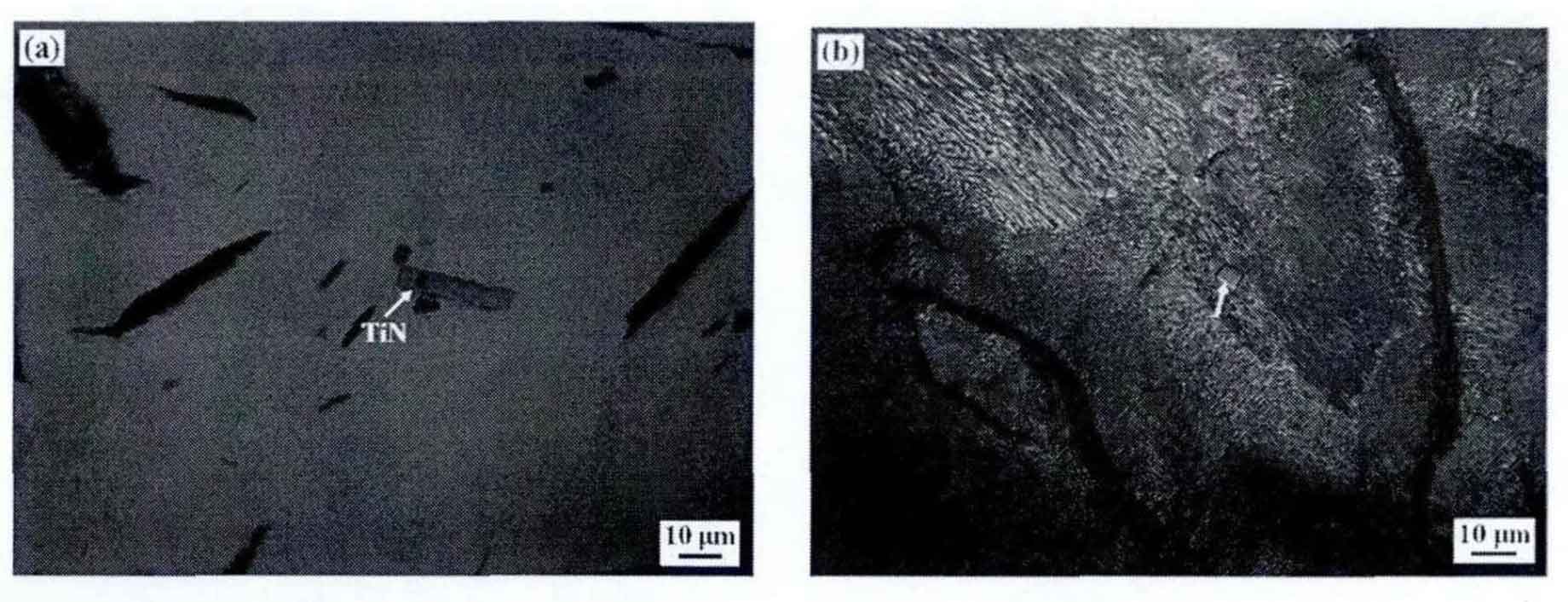
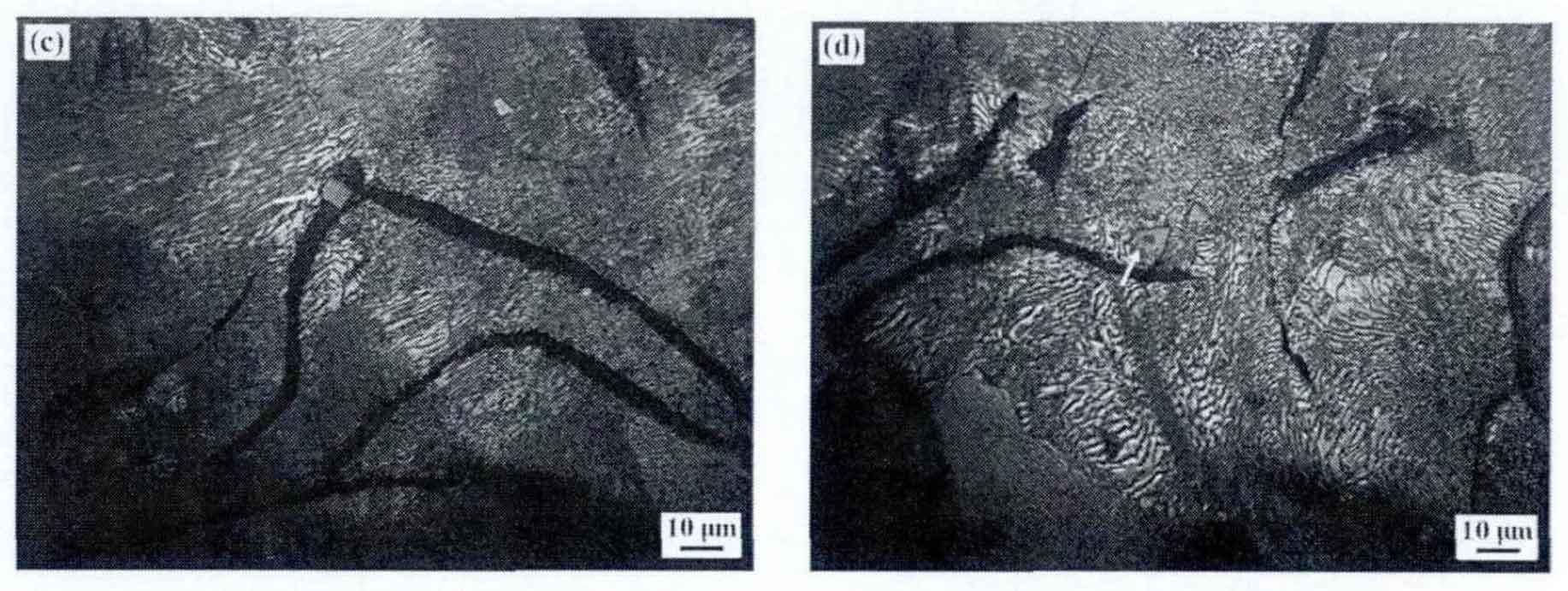
In the melting process of gray cast iron, the larger tin particles in the raw and auxiliary materials failed to melt completely, but remained in the melt. Therefore, according to the dissolution precipitation mechanism, small tin particles will continue to dissolve and diffuse, and large tin particles will further grow. Analyzing the growth process of various tin, the larger tin in figure (a) is caused by the heredity of raw materials, and the fine tin particles in figure (b) – (d) are mainly formed from supercooled molten iron or supercooled austenite through crystallization or crystallization process. Therefore, through analysis, the formation thermodynamics of tin crystallization and crystallization process in gray cast iron is as follows:
The crystallization of tin in supercooled melt is mainly formed by the reaction of molten mirror atom [Ti] and nitrogen atom [n]. The reaction process is as follows:

According to the formula:

Due to the low content of Ti in gray cast iron, it can be regarded as a thin solution, and the values of FTI and FN are approximately 1. Brought in by the formula, you can get:

Thus, when tin inclusion phase crystallizes in gray iron solder, [%ti] [%n] relationship between solubility product and temperature T.

Kunze obtained [%ti]] when tin crystallization was formed from undercooled austenite through thermodynamic calculation [%n] the relationship between fusion product and temperature T is:

According to the formula, combining the melting temperature of 1510 ° C, pouring temperature of 1420 ° C, liquidus temperature of 1210 ° C and solidus temperature of 1120 ° C in the actual production of gray cast iron, we can obtain [%11] Relation curve between [%n].
